Number of views:
1000
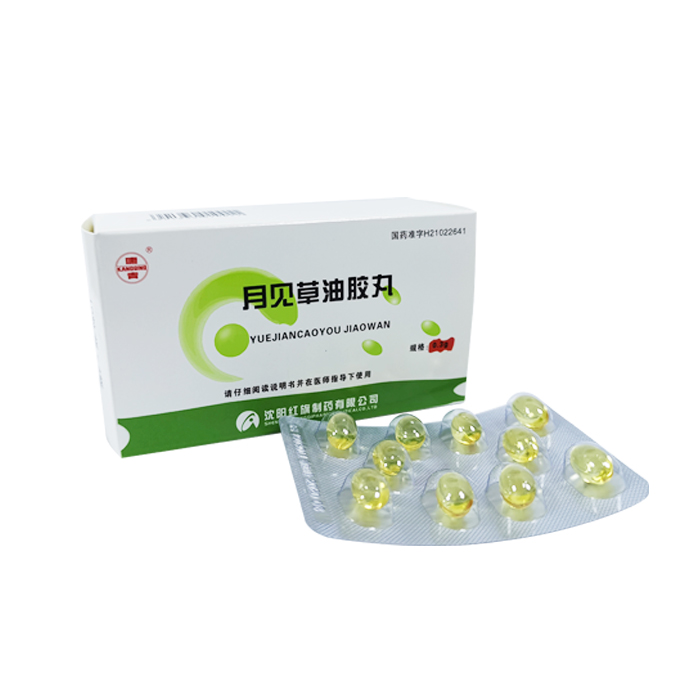
Oenothera Biennis Oil Soft Capsules
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Quantity
-
+
Stock:
Category
1
Product description
Parameters
Instructions for evening primrose oil capsules
Please read the instructions carefully and use under the guidance of a doctor

【Drug Name】
Generic name: Evening primrose oil capsules
English name: Oenothera Biennis Oil Soft Capsules
Chinese Pinyin: Yuejiancaoyou Jiaowan
【Ingredients】
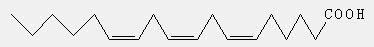
Chemical name: All-cis 6,9,12-octadecatrienoic acid.
Chemical Structure:
【Properties】
This product is a capsule, the content is light yellow to orange yellow oily liquid.
【Indications】
Used to prevent arteriosclerosis, reduce blood fat, etc.
【specification】
0.3g (based on evening primrose oil)
【Dosage】
oral.
5-6 capsules at a time, 2 times a day, or as directed by a doctor.
【Adverse reactions】
Individual patients may experience nausea, loose stools and other adverse reactions at the initial stage of oral medication, which may get better with continued medication.
【Taboo】
1. People who are allergic to this product are prohibited.
2. Disabled for patients with bleeding disorders.
3. Prohibited for pregnant women.
【Precautions】
If the character changes, the color is brown and cannot be applied.
[Medicine for pregnant and lactating women]
Prohibited for pregnant women.
【Children's Medication】
uncertain.
【Medications for the Elderly】
uncertain.
【medicine interactions】
uncertain.
【Overdose】
uncertain.
【Pharmacology and Toxicology】
1. Pharmacology
This product is rich in γ-linolenic acid (GLA), which can be converted into PEG2, PGI2, PGF2a, and has obvious effects of lowering blood lipids and anti-platelet aggregation. γ-linolenic acid has an obvious cholesterol-lowering effect, and its effectiveness is 163 times that of linoleic acid. Evening primrose oil can reduce serum total cholesterol, low-density lipoprotein cholesterol, and very low-density lipoprotein cholesterol in high-fat rats, and at the same time significantly increase high-density cholesterol levels. The change in high-density lipoprotein cholesterol was due to the increase in the cholesterol level of the high-density lipoprotein sub-component, and the cholesterol level outside the high-density lipoprotein sub-component did not change significantly. Wistar rats were given hyperlipidemia styling agent or ethyl sulfuric acid to create a pathological model of hyperlipidemia. The results showed that evening primrose oil and its sodium salt have strong hypolipidemic and anti-atherosclerotic effects. This product can participate in the synthesis and balance of prostaglandins. It can still significantly inhibit platelet aggregation and platelet synthesis thromboxane A2 (TXA2), thereby changing the ratio of TXA2/PGI2, and has the effect of preventing and treating thrombotic cardiovascular diseases. γ-linolenic acid has a stimulating effect on brown adipose tissue, promotes the activity of brown fatty acid mitochondria, consumes too much calories to achieve a certain weight loss effect.
2. Toxicology
The acute toxicity test of evening primrose oil failed to determine the LD50 of mice. Rabbits were killed after continuous administration of 3g/kg twice a day for 120 days. No heart, liver, kidney, spleen, adrenal glands, or adrenal glands were found. Any pathological changes in the thymus.
【Pharmacokinetics】
Gamma-linolenic acid (GLA) is one of the essential fatty acids of the human body. It can be metabolized into di-homo-γ-linolenic acid (DGLA) or arachidonic acid (AA) in the body, and then converted into prostaglandins (PGs) and white triglycerides. Ene (LT). γ-linolenic acid (GLA) and its series of metabolites have extensive physiological effects in the immune system, cardiovascular system, reproductive system, and endocrine system.
【Storage】
Shade, seal, and store in a dry place.
【package】
Aluminum-plastic blister packaging, 10 tablets/plate; carton packaging, 4 plates/box.
Aluminum-plastic blister packaging, 10 tablets/plate; carton packaging, 5 plates/box.
【Validity Period】
Tentative 18 months
【Executive Standard】
State Food and Drug Administration National Drug Standard WS-10001-(HD-0599)-2002
【Approval Number】
National Medicine Standard H21022641
【manufacturer】
Company Name: Shenyang Hongqi Pharmaceutical Co., Ltd.
Production address: No. 6, Xinluo Street, Hunnan New District, Shenyang City
Postal Code: 110179
Phone number: (024) 23786260 23786261
Fax number: (024) 23786263
Scan the QR code to read on your phone
Scan the QR code to read on your phone
Previous
None
相关产品


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263