Number of views:
1000
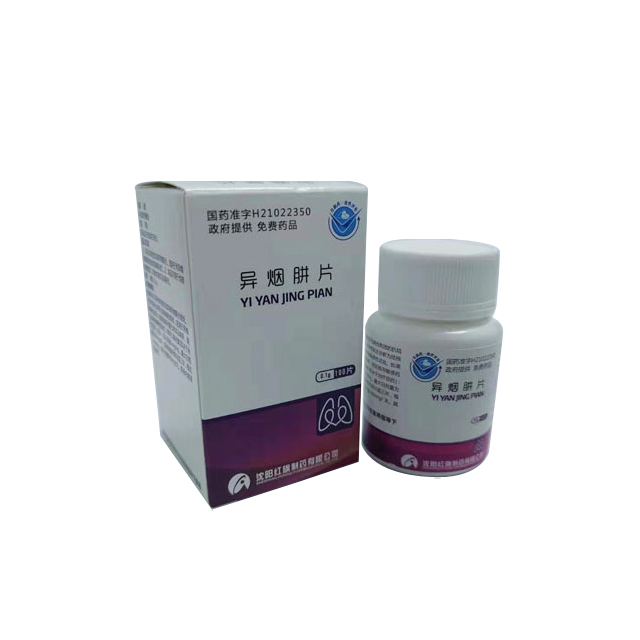
Isoniazid Tablets(0.1g)
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Quantity
-
+
Stock:
Category
1
Product description
Parameters
Instructions for Isoniazid Tablets
Please read the instructions carefully and use under the guidance of a doctor
Warning: There have been reports of serious and even fatal drug-induced liver damage caused by isoniazid treatment, which may even occur several months after the end of treatment. Daily drinking can increase the risk of drug-induced liver injury caused by isoniazid, and there is no accurate data on the death rate of drug-induced liver injury caused by isoniazid. Therefore, patients taking isoniazid should be closely monitored and followed up monthly.
Drug-induced liver injury usually occurs 3 months before isoniazid treatment. In the case of continuous medication, enzyme levels will usually return to normal levels, but some patients will experience progressive liver damage. The high-risk factors for drug-induced liver injury are: daily alcohol consumption, chronic liver disease, and drug injection. A recent study showed that female patients taking isoniazid have an increased risk of fatal liver damage; women may also have an increased risk of drug-induced liver damage after childbirth. Therefore, the above population should be monitored more closely, such as increasing the number of laboratory tests. If the abnormal value of liver function index exceeds 3 to 5 times the upper limit of normal value, isoniazid should be considered to be stopped. Liver function tests cannot replace regular monthly clinical assessments, otherwise adverse reactions during treatment cannot be assessed on time. Patients should be instructed to immediately report signs or symptoms related to liver injury or other adverse reactions, including any of the following conditions: unexplained anorexia, nausea, vomiting, dark urine, jaundice, rash, persistent hand and foot paresthesia, persistent fatigue , Fatigue or fever that lasts more than 3 days and/or abdominal tenderness, especially when the upper right abdomen is uncomfortable. If the patient has the above symptoms or the test results suggest liver damage, isoniazid should be stopped immediately. Studies have shown that continued use of the drug in such cases will cause more serious liver damage.
When isoniazid-related liver injury occurs in pulmonary tuberculosis patients, other anti-tuberculosis drugs can be used as an alternative treatment. If you need to use isoniazid again, you should use it after the symptoms disappear and the laboratory abnormalities return to normal. When the patient is re-treated with isoniazid, the initial dose should be as small as possible, and then gradually increase the dose. If there are any signs of recurrence of liver disease, the drug should be stopped immediately.
Patients with acute liver disease should postpone the time of preventive treatment.
【Drug Name】
Generic name: Isoniazid tablets
English name: Isoniazid Tablets
Chinese Pinyin: Yiyanjing Pian
【Ingredients】
The main ingredient of this product is isoniazid.
Chemical name: 4-Pyridinecarboxamide
Chemical Structure:
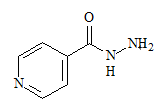
Molecular formula: C 6 H 7 N 3 O
Molecular weight: 137.14
【Properties】
This product is white or almost white film.
【Indications】
In order to ensure the effectiveness of this product and other antibacterial drugs and reduce the occurrence of drug resistance, the antibacterial drug treatment plan can be changed or adjusted according to the results of sputum culture and drug sensitivity. If such data are lacking, treatment will be given based on the results of local epidemiology and drug susceptibility experience.
1. This product is combined with other anti-tuberculosis drugs and is suitable for the treatment of various types of tuberculosis sensitive to isoniazid.
2. This product can be used for the prevention of tuberculosis, see the following for details (the tuberculosis skin test (PPD) standard is in brackets):
①Patients infected with human immunodeficiency virus (HIV) (≥5mm) and persons suspected of being HIV-infected (it is not yet certain to be infected with HIV but highly suspected). Preventive treatment of HIV-infected patients should be continued for at least 12 months.
②Persons (≥10mm) who have close contact with patients who have been newly diagnosed with infectious tuberculosis.
③Patients who have recently turned positive or have progressed from PPD: results of tuberculin skin test (increased by ≥10~15mm within 2 years). All infants and young children younger than 5 years old and skin test results> 10mm are included.
④The chest X-ray of the patient (≥10mm) is abnormal, suggesting that there may be a healed fibrotic lesion caused by Mycobacterium tuberculosis in the lungs. Patients with tuberculosis fibrosis healing or silicosis can be considered for preventive treatment, using isoniazid for 12 months or a combination of isoniazid and rifampicin for 4 months.
⑤ HIV-negative (>10mm) intravenous drug users.
⑥ People at high risk of tuberculosis (≥10mm): silicosis, diabetes, long-term use of adrenal cortex hormone therapy, immunosuppressive therapy, some blood and reticuloendothelial diseases such as leukemia or Hodgkin's disease, advanced kidney disease, sudden weight loss Or clinical conditions related to chronic malnutrition (including: intestinal bypass surgery for obesity, post-gastrectomy status (with or without weight loss), chronic peptic ulcer disease, chronic malabsorption syndrome, and oropharyngeal or upper gastrointestinal tract Nutrient intake disorder caused by cancer). The above population may consider preventive treatment, using isoniazid for 12 months or a combination of isoniazid and rifampicin for 4 months.
3. According to domestic clinical experience, this product is combined with other anti-tuberculosis drugs and can also be used for other mycobacterial infections, but there is no sufficient clinical research data to support it.
【Specifications】 0.1g
【Dosage】
1. Treatment of tuberculosis
Isoniazid should be used in combination with other effective anti-tuberculosis drugs. All patients newly diagnosed with tuberculosis should be tested for drug susceptibility. If Mycobacterium tuberculosis is resistant to this product, it should be treated with sensitive drugs.
Common oral dosage (depending on the purpose of treatment):
adult
Take 5mg/kg daily, the maximum daily dose is 300mg/day;
Or take the medicine twice or three times a week, 15 mg/kg each time, and the maximum dose is 900 mg/day.
child
Take 10mg/kg~15mg/kg daily, the maximum daily dose is 300mg/day;
Or take the medicine twice or three times a week, each time 20mg/kg ~ 40mg/kg, the maximum dose is 900mg/day.
Tuberculosis patients who are not infected with HIV
There are three initial treatment options for children and adults with tuberculosis:
Alternative 1: Daily maintenance therapy with isoniazid, rifampicin and pyrazinamide for 8 weeks, and then maintenance therapy with isoniazid and rifampicin 2 to 3 times a day or a week for 16 weeks. The initial treatment regimen should be combined with ethambutol or streptomycin until the patient is confirmed to be sensitive to isoniazid and rifampicin. If the relative prevalence of isoniazid-resistant Mycobacterium tuberculosis in the community is less than or equal to 4%, the fourth drug can be optionally added.
Option 2: Daily use of isoniazid, rifampicin, pyrazinamide and streptomycin or ethambutol for maintenance treatment for 2 weeks, and then adjust the frequency of medication to 2 times a week for maintenance treatment for 6 weeks, and finally The combined use of isoniazid and rifampicin was administered twice a week for maintenance treatment for 16 weeks.
Alternative 3: Combination of isoniazid, rifampicin, pyrazinamide and ethambutol or streptomycin for maintenance treatment for 6 months, three times a week.
All treatment plans recommend the use of direct face-to-face supervised treatment (see DOT for details).
The above treatment options are only suitable for tuberculosis that is sensitive to standard anti-tuberculosis drugs. Ethambutol is not recommended for children who cannot monitor changes in vision.
Tuberculosis patients infected with HIV
When the immune function is impaired, the anti-tuberculosis curative effect of tuberculosis patients may not be as good as those with normal immune function. Therefore, the anti-tuberculosis treatment plan for such patients should be individualized. Such patients may have poor drug absorption and need to monitor blood drug concentration, especially in patients with advanced HIV, to prevent the emergence of multidrug-resistant tuberculosis (MDR-TB).
Patients with extrapulmonary tuberculosis
The basic principles of tuberculosis treatment also apply to extra-pulmonary tuberculosis. Miliary tuberculosis, bone/joint tuberculosis and tuberculous meningitis should be treated for at least 12 months.
The efficacy of extrapulmonary tuberculosis is mainly evaluated based on clinical and imaging results.
Compared with pulmonary tuberculosis, extrapulmonary tuberculosis often requires auxiliary treatment such as surgery and corticosteroids. For example, surgical biopsy confirms the diagnosis, surgical removal of the thickened pericardium, and relief of related spinal cord compression. Studies have shown that the early application of corticosteroids can help prevent tuberculous pericarditis from developing into constrictive pericarditis and reduce the neurological sequelae of tuberculous meningitis at various stages.
Patients with tuberculosis complicated by pregnancy
The treatment plan for pregnant patients must be adjusted. It is recommended to consult a tuberculosis expert to formulate corresponding treatment plans at different stages of pregnancy.
Treatment of patients with multidrug-resistant tuberculosis (MDR-TB)
Patients with multidrug-resistant tuberculosis (ie, at least resistant to isoniazid and rifampicin) are difficult to treat. Individualized treatment must be implemented based on the results of drug sensitivity tests. In this case, it is recommended to consult a tuberculosis expert to develop a treatment plan.
Direct Observation of Supervised Therapy (DOT)
The main cause of drug-resistant tuberculosis is that the patient does not follow the doctor's advice. The use of DOT can ensure patient compliance with medication. DOT is when medical staff or other persons in charge observe patients taking anti-tuberculosis drugs. DOT can be used in various treatment options and is recommended for all patients. 2. Prevention and treatment of tuberculosis
Before the use of isoniazid preventive treatment, positive tuberculosis must be ruled out or active pulmonary tuberculosis must be ruled out through chest imaging. If extrapulmonary tuberculosis is suspected, proper evaluation should be performed first.
Adults over 30kg: Take 300mg daily.
Infants and children: take 10mg/kg daily (maximum daily dose 300mg). When compliance with daily preventive treatment cannot be ensured, it is recommended to use the DOT method, taking the drug twice a week, 20-30mg/kg each time (the highest dose is 900mg).
Because the recurrence rate is higher after stopping treatment too early, taking isoniazid for a full course of treatment is an important part of the treatment plan. When drug-resistant bacteria appear during treatment, the treatment plan may need to be changed.
It is recommended that patients with malnutrition and neuropathy risk (such as alcoholics and diabetic patients) take vitamin B6 at the same time.
【Adverse reactions】
The most common adverse reactions are nervous system reactions and liver damage.
Nervous system: The most common reaction is peripheral nervous system disease. The incidence of this adverse reaction is related to the dose, and is most common in patients with malnutrition and neuropathy risk (such as alcohol abuse and diabetes). The common early reaction is hand-foot paresthesia, and the incidence of "slow acetylation" is higher. The rare neurotoxic side effects at regular doses include: convulsions, toxic encephalopathy, optic neuritis, optic atrophy, memory impairment, and toxic psychosis.
Liver system: elevated serum transaminase, elevated serum bilirubin, jaundice, occasional severe liver damage and even fatal hepatitis. The common prodromal symptoms of hepatitis are anorexia, nausea, vomiting, fatigue, malaise and fatigue. 10% to 20% of patients taking isoniazid have a transient mild increase in serum transaminase levels. This abnormality usually appears in the first 1 to 3 months of treatment, but it may also occur at any time during the treatment. In most cases, liver enzyme levels will return to normal, so it is usually not necessary to stop the drug when there is a mild increase in serum transaminase. But in some cases, liver damage can progress sexually. If the AST value exceeds 3 to 5 times the upper limit of normal, it is strongly recommended to stop the drug. The incidence of liver damage increases with age.
Gastrointestinal reactions: nausea, vomiting, epigastric pain and pancreatitis.
Blood system: agranulocytosis, hemolysis, sideroblasts or aplastic anemia, thrombocytopenia and eosinophilia.
Hypersensitivity reactions : fever, skin rash (measles, maculopapular rash, purpura or exfoliative dermatitis), lymphadenopathy and vasculitis, toxic epidermal necrolysis, drug eruption with eosinophilia and systemic symptoms (DRESS).
Metabolism and endocrine system: Vitamin B6 deficiency, pellagra, hyperglycemia, metabolic acidosis and gynecomastia.
Other: rheumatic syndrome and systemic lupus erythematosus-like syndrome.
【Taboo】
This product is contraindicated in patients with severe hypersensitivity to isoniazid, including drug-induced hepatitis; past liver damage related to isoniazid; severe adverse reactions to isoniazid such as drug-induced fever, chills, and arthritis ; Any type of acute liver disease.
【Precautions】
1. General precautions
(1) If hypersensitivity occurs when using this product, the drug should be discontinued and evaluated immediately. If you need to continue using isoniazid, you should use it after the symptoms disappear. When the patient restarts isoniazid treatment, the initial dose should be as small as possible, and then gradually increase the dose. If there are any signs of recurrence of hypersensitivity, the drug should be stopped immediately.
(2) The following patients should be closely monitored when using isoniazid:
1) Patients who drink alcohol daily. Patients who drink alcohol daily are more likely to develop isoniazid-related liver damage.
2) Patients with chronic active hepatitis or severe renal insufficiency.
3) Patients who need to take other drugs for a long time during treatment.
4) Patients with a history of discontinuation of isoniazid in the past.
5) Patients with peripheral neuropathy or at risk of neuropathy.
6) Pregnancy.
7) Injecting drug users.
8) A small number of female patients, especially postpartum women.
9) HIV positive persons.
(3) Isoniazid can also be used for the treatment of other mycobacterial infections, and a treatment plan needs to be formulated according to the results of bacterial identification and drug sensitivity experiments.
2. Laboratory inspection
Isoniazid has a higher incidence of drug-induced liver injury in some special populations, including daily alcohol consumption, chronic active hepatitis, injection drug use, and a small number of female patients, especially postpartum women. The above patients should check liver function regularly before and during treatment. Liver function monitoring should be performed monthly during preventive treatment, and the number of monitoring can be increased if necessary. If the transaminase level exceeds the upper limit of normal value by 3 to 5 times, the drug should be suspended.
[Medicine for pregnant women and lactating women]
(1) When rats and rabbits were given isoniazid orally during pregnancy, embryonic death was observed. Reproductive toxicity studies in mice, rats and rabbits show that isoniazid has no teratogenic effects. There are no adequate and reliable clinical studies on pregnant women. When pregnant women are in active tuberculosis infection period, the benefit of mothers using isoniazid treatment will be greater than the risk to the fetus, so isoniazid treatment is recommended. When using isoniazid for preventive treatment, the possible benefits of preventive treatment should be fully weighed against the potential risks to the fetus. Preventive treatment should generally be started after delivery to reduce the risk of fetal exposure to isoniazid; the level of isoniazid in breast milk is low and will not cause harm to the newborn. Isoniazid can penetrate the placental barrier, so newborns exposed to isoniazid through their mothers should be closely monitored for adverse reactions.
(2) A small amount of isoniazid in breast milk will not be toxic to newborns; therefore, breastfeeding can still be continued while taking isoniazid. The content of isoniazid in breast milk is very low, not enough to prevent or treat infants during lactation.
【Children's Medication】
Strictly follow the usage and dosage of children.
【Geriatric Medication】
The incidence of drug-induced liver injury in elderly patients is increased.
【medicine interactions】
Food: Isoniazid should not be taken with food. Studies have shown that when isoniazid is taken with food, its bioavailability is significantly reduced. Patients receiving isoniazid treatment should avoid foods rich in tyramine and histamine. Since isoniazid slightly inhibits monoamine oxidase activity, it may interact with foods containing tyramine (cheese, red wine). Isoniazid can also inhibit diamine oxidase. Patients who consume histamine-rich foods (such as bonito, tuna, and other tropical fish) during medication are prone to histamine poisoning symptoms (such as headache, sweating, palpitations, flushing, and hypothermia). blood pressure).
Drinking: Drinking alcohol daily while taking isoniazid can easily cause liver toxicity induced by this product and accelerate the metabolism of isoniazid. Therefore, it is necessary to adjust the dose of isoniazid and closely observe signs of liver toxicity. Patients should be advised to avoid drinking alcohol and alcoholic beverages during the medication.
Acetaminophen: There have been reports of severe acetaminophen toxic reactions in patients taking isoniazid. Studies have found that this toxicity may be caused by an undiscovered interaction between isoniazid and acetaminophen, and the molecular mechanism of this interaction has been proposed. However, current research shows that isoniazid can induce the activity of the multifunctional oxidase P-450IIE1 in the liver to convert acetaminophen into toxic metabolites. Studies have shown that after pretreatment with isoniazid in rats, the liver toxicity of acetaminophen increases.
Carbamazepine : Isoniazid can inhibit the metabolism of antiepileptic drugs and increase the blood concentration. During the medication, the signs and symptoms related to carbamazepine toxicity should be closely monitored, and the dose of carbamazepine should be adjusted according to the situation.
Phenytoin: Isoniazid can cause the blood concentration of phenytoin to increase. In order to avoid phenytoin poisoning, the dosage of anticonvulsants should be adjusted appropriately.
Sodium valproate: A recent case study showed that the blood level of valproic acid may increase when combined with isoniazid. When isoniazid and sodium valproate are used in combination, the blood concentration of valproic acid should be monitored and the dose of sodium valproate should be adjusted appropriately.
Ketoconazole: Antifungal drugs such as ketoconazole and isoniazid may have potential interactions. Studies have shown that after taking isoniazid and rifampicin for 5 months, the AUC of ketoconazole was reduced by 88%.
Miconazole: Isoniazid should not be combined with miconazole, because it can reduce the latter's blood concentration.
Itraconazole: It has been reported that isoniazid has the effect of inducing liver drug-metabolizing enzymes, thereby promoting the metabolism of itraconazole, leading to a decrease in its blood concentration.
Theophylline: A recent study showed that the combined use of isoniazid and theophylline may increase the blood concentration of theophylline. In some cases, the clearance rate of isoniazid may also decrease slightly. Due to the narrow therapeutic range of theophylline, the blood concentration of theophylline should be closely monitored and the dosage of theophylline should be adjusted appropriately.
Aluminum-containing antacids: Aluminum-containing antacids can delay and reduce the absorption of oral isoniazid and reduce the blood concentration, so you should avoid taking both at the same time, or take isoniazid at least 1 hour before oral antacids.
Anticoagulant: When an anticoagulant (such as coumarin or indandione derivatives) is used at the same time with isoniazid, the anticoagulant effect is enhanced by inhibiting the enzymatic metabolism of the anticoagulant.
Anti-tuberculosis drugs: Isoniazid and cycloserine can increase central nervous system adverse reactions (such as dizziness or drowsiness). The dose should be adjusted and the signs of central nervous system toxicity should be closely observed, especially for work that requires high sensitivity. Of patients. When isoniazid is used in combination with rifampicin, ethionamide, etc., it can increase the risk of liver toxicity, especially in patients with liver damage or rapid isoniazid acetylation, so in the first 3 months of treatment Close follow-up should be followed for signs of liver toxicity.
Adrenocortical hormone: especially when combined with prednisolone, it can increase the metabolism and excretion of isoniazid in the liver, resulting in lower plasma concentration of the latter and affecting the curative effect. It is more significant in patients with rapid acetylation, and the dosage should be adjusted appropriately. .
Alfentanil : When combined with alfentanil, isoniazid is a liver drug enzyme inhibitor, which can prolong the effect of alfentanil.
Disulfiram : combined with disulfiram can enhance the central nervous system, causing dizziness, uncoordinated movements, irritability and insomnia, etc.; combined with enflurane can increase the effects of nephrotoxic inorganic fluoride metabolites form.
【Overdose】
Symptoms and signs: Symptoms appear within 30 minutes to 3 hours after taking an overdose of isoniazid. Early symptoms include nausea, vomiting, dizziness, slurred speech, blurred vision, and hallucinations (including bright colors and strange patterns). In severe overdose, respiratory distress and central nervous system depression may occur, from trance to deep coma, accompanied by severe intractable seizures. Typical laboratory abnormalities after isoniazid poisoning include: severe metabolic acidosis, ketonuria, and hyperglycemia.
Treatment: Overdose of isoniazid 80mg/kg to 150mg/kg, if left untreated or not treated in time, can lead to neurotoxicity and even death, but most patients overdose this product within a few hours after receiving appropriate treatment and healing well .
For asymptomatic patients: activated carbon can reduce the absorption of isoniazid in the gastrointestinal tract. Asymptomatic patients should also be gastric lavage. When taking the above-mentioned treatment measures, care should be taken to keep the patient's breathing smooth. Patients with acute intake of >80 mg/kg isoniazid should be given an equal dose (g) of vitamin B6 intravenously. If the intake dose of isoniazid is not clear, adults should receive 5 g of vitamin B6 intravenously within 30 to 60 minutes, and children can receive 80 mg/kg of vitamin B6 intravenously.
For symptomatic patients: when treating seizures and reducing the absorption of isoniazid, adequate ventilation should be ensured, cardiac output maintained and airway protected. If the intake dose of isoniazid is known, the same dose (g) of vitamin B6 should be given by a slow intravenous bolus for 3 to 5 minutes. If the dose of isoniazid intake is unknown, 5g vitamin B6 is injected intravenously for adults and 80mg/kg vitamin B6 for children. If the attack continues, vitamin B6 can be repeated as needed. Very few patients need to be given more than 10g of vitamin B6. The maximum safe dose of vitamin B6 in the treatment of isoniazid poisoning is unclear. If the patient's use of vitamin B6 is not effective, diazepam can be used; but phenytoin sodium should be used with caution, because isoniazid can interfere with the metabolism of phenytoin sodium.
General situation: Collect patient blood samples, immediately perform blood gas analysis, check electrolyte levels, BUN, blood sugar, etc.; prepare corresponding plasma blood and perform blood type cross matching to prepare for possible hemodialysis.
Rapid control of metabolic acidosis: Patients with a degree of isoniazid poisoning may experience hypoventilation. Administration of sodium bicarbonate at this time can cause hypercapnia. If the patient has respiratory insufficiency, the ventilation must be carefully monitored by monitoring the blood carbon dioxide level, and mechanical ventilation support must be given.
Dialysis: Both peritoneal dialysis and hemodialysis can be used for the treatment of isoniazid overdose. If treatment with vitamin B6, diazepam and sodium bicarbonate can alleviate epilepsy and acidosis, peritoneal dialysis and hemodialysis are not needed.
In addition to timely and repeated blood gas analysis and monitoring and laboratory examinations, close respiratory treatment and other intensive care measures should also be taken to prevent hypoxia, hypotension, aspiration, and pneumonia.
【Pharmacology and Toxicology】
Pharmacological action
Mechanism
Isoniazid inhibits the synthesis of mycolic acid, a key component of bacterial cell walls. At therapeutic concentrations, isoniazid has a bactericidal effect on both intracellular and extracellular Mycobacterium tuberculosis in the growth and reproduction phase.
Resistance
The resistance of isoniazid is usually caused by genetic mutations in katG, inhA, kasA and ahpC. When isoniazid monotherapy is administered, Mycobacterium tuberculosis quickly develops drug resistance.
Microbiological test
Two standardized in vitro susceptibility test methods can be used to detect the sensitivity of isoniazid to Mycobacterium tuberculosis. Agar ratio method (CLSI, M24-A2): Using Middlebrook 7H10 or 7H11 medium, adding final concentrations of 0.2μg/mL and 1.0μg/mL isoniazid respectively; Mycobacterium tuberculosis is diluted to 10 with McDaniel's turbidity standard 0.5~1.0 -2 to 10-4 times. MIC99 was calculated by comparing the amount of bacteria grown in the drug-containing medium and the control medium. The growth of mycobacteria in the presence of the drug is ≥1% compared with the control, indicating drug resistance.
Radioactive broth method: Use the BACTEC 460 to compare the growth index (GI) of the drug-free medium and the medium containing 0.2μg/mL and 1.0μg/mL isoniazid. The test needs to strictly abide by the manufacturer's sample processing method and data analysis.
Mycobacterium tuberculosis MIC99 value ≤ 0.2μg/mL is considered to be sensitive to isoniazid. Unless the same drug concentration is evaluated, the sensitivity test results obtained by the above two methods cannot be compared.
The clinical relevance of the in vitro susceptibility of Mycobacterium species other than Mycobacterium tuberculosis using BACTEC or the ratio method has not been determined.
Toxicology Research
Genotoxicity: Isoniazid is weakly mutagenic in the TA100 and TA1535 strains of Salmonella typhimurium (Ames test) that are not metabolically activated.
Reproductive toxicity: oral administration of isoniazid in rats and rabbits during pregnancy can cause embryonic death. Reproductive toxicity studies in mice, rats and rabbits did not show teratogenic effects of isoniazid.
Carcinogenicity: Isoniazid can induce lung tumors in a variety of mouse strains. It has not been proven that isoniazid has carcinogenic effects on humans. (Note: A child who was exposed to isoniazid prenatally has been diagnosed with mesothelioma. This patient has no other obvious carcinogenic risk factors).
【Pharmacokinetics】
1-2 hours after the oral administration of this product, the plasma concentration reaches its peak and drops to 50% or less within 6 hours. Isoniazid easily spreads to all body fluids (cerebrospinal fluid, pleural fluid and ascites), tissues, organs and excrement (saliva, sputum and feces), and can also pass through the placental barrier and enter breast milk at the same concentration as the blood concentration. Within 24 hours, 50%-70% of isoniazid is excreted in urine.
Isoniazid is mainly metabolized through acetylation and deshydrazine reactions. The rate of acetylation is determined by genes. About 50% of blacks and whites are "slow inactivators", and the rest are "fast inactivators"; most Eskimos And Orientals are "quick killers".
The rate of acetylation does not significantly change the effectiveness of isoniazid, but slow acetylation may lead to higher blood drug levels and therefore increase toxic reactions.
Adults sometimes experience pyridoxine (vitamin B6) deficiency when taking isoniazid in large doses, which may be due to the competitive binding of deoxyinase with pyridoxal phosphate.
[Storage] shading, sealed, and stored in a dry place.
[Packing] Oral solid medicinal high-density polyethylene bottle, 100 tablets/bottle.
[Validity] 24 months
【Executive standard】 YBH05632019
[Approval number] National Medicine Zhunzi H21022350
【manufacturer】
Company Name: Shenyang Hongqi Pharmaceutical Co., Ltd.
Production address: No. 6, Xinluo Street, Hunnan New District, Shenyang City
Postal Code: 110179
Phone number: (024) 23786260, 23786261
Fax number: (024) 23786263
Website: www.hongqipharma.com
Scan the QR code to read on your phone
Scan the QR code to read on your phone
Previous
Rifampicin for Injection(0.45g)
相关产品


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263