Number of views:
1000
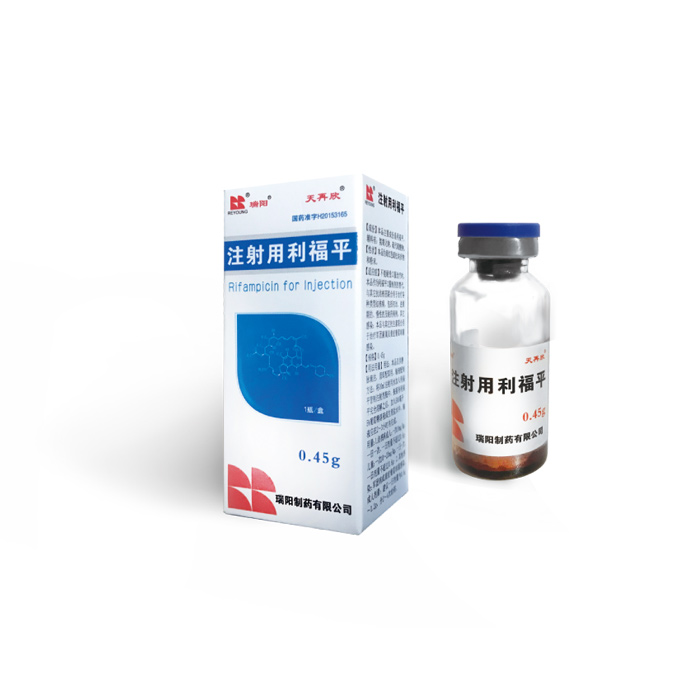
Rifampicin for Injection(0.45g)
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Quantity
-
+
Stock:
Category
1
Product description
Parameters
Tian Zaixin® Ruiyang®
Instructions for Rifampicin for Injection
Please read the instructions carefully and use it under the guidance of a physician.
【Drug Name】
Generic name: Rifampicin for Injection
English name: Rifampicin for Injection
Chinese Pinyin: Zhusheyong Lifuping
[Ingredients] The main ingredient of this product is rifampicin, and its chemical name: 3-[[ (4-methyl-1-piperazinyl)imino]methyl]-rifamycin.
The chemical structural formula is:
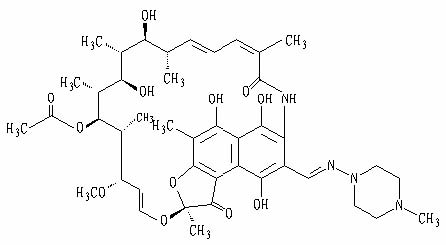
Molecular formula: C 43 H 58 N 4 O 12
Molecular weight: 822.95
Excipients are: sodium hydroxide, sodium thiosulfate
[Properties] This product is dark red loose lumps and powder.
【Indications】
When oral treatment cannot be tolerated, this product can be used as an alternative to oral preparations of rifampicin. It is used in combination with other anti-tuberculosis drugs to treat various types of tuberculosis, including newly-treated, advanced, chronic and drug-resistant cases.
Other infections: This product is used in combination with other antibiotics to treat Legionella and Severe Staphylococcus infections.
【Specifications】 0.45g
【Dosage】
usage:
This product is for intravenous infusion only, and it should be used as soon as possible. Infusion preparation method: add 10ml of water for injection into the rifampicin control injection bottle, shake until rifampicin is completely dissolved, add 500ml of 5% dextrose solution or normal saline, the infusion should be completed within 2 to 3 hours.
Dosage:
1. Tuberculosis
Adults: 10mg/kg once, once a day, the daily dose does not exceed 0.6g;
Children: 10~20mg/kg once, once a day, the daily dose does not exceed 0.6g;
2. Other infections: Legionnaires' disease or severe staphylococcal infection.
Adult dose: The recommended daily dose is 0.6g~1.2g, divided into 2 to 4 times.
【Adverse reactions】
Digestive system: Heartburn, epigastric discomfort, anorexia, nausea, vomiting, jaundice, flatulence, colic and diarrhea may occur. In vitro experiments have shown that Clostridium spp. is sensitive to rifampicin. There are also reports that pseudomembranous colitis is related to the use of rifampicin (and other broad-spectrum antibiotics). Such patients have diarrhea associated with antibiotic use.
Liver: This product has hepatotoxicity. It usually occurs when it is used in combination with other anti-tuberculosis drugs. It is manifested as elevated ALT, hepatomegaly, and jaundice in severe cases.
Blood system: High-dose intermittent treatment can cause thrombocytopenia. However, with reasonable daily supervision and treatment, this rarely happens. Thrombocytopenia and purpura can be restored after stopping the drug. When purpura occurs and continues to use rifampin after recovery, occasional cerebral hemorrhage and death have been reported. There are rare reports of diffuse intravascular coagulation. There are occasional reports of transient leukopenia, hemolytic anemia, and decreased hemoglobin. Agranulocytosis is rarely reported. There are rare reports of diffuse intravascular coagulation.
Central nervous system: headache, fever, drowsiness, fatigue, ataxia, dizziness, inattention, confusion, behavior changes, muscle weakness, pain and general numbness. There are few reports of mental illness.
Ophthalmology: Visual impairment may occur.
Endocrine system: Menstrual disorders may occur. There are occasional reports of adrenal insufficiency.
Kidney: Can cause BUN and blood uric acid to increase. Occasionally, hemolysis, hemoglobinuria, hematuria, interstitial nephritis, acute tubular necrosis, renal insufficiency and acute renal failure. These are usually considered to be caused by a high degree of allergic reactions, which usually occur after intermittent treatment or after deliberate or accidental interruption of daily treatment. These symptoms can be restored after stopping medication or starting rational medication.
Skin: Skin reactions are generally mild and self-limiting, including skin redness, itching, with or without the appearance of rashes. Severe skin reactions are mostly caused by allergic reactions, but they are not common.
Allergic reactions: occasional itching, urticaria, skin rash, pemphigoid reaction, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrosis, vasculitis, eosinophilia, oral pain , Mouth and tongue sores and conjunctivitis. Few reports of allergic reactions.
Others: swelling of the face and limbs. Intermittent treatment can also cause flu-like symptoms (such as fever, chills, headache, dizziness, bone pain), shortness of breath, lowered blood pressure, and shock. If the patient fails to take the drug on time every day or resumes the drug after a certain interval, "flu-like syndrome" may also occur.
【Taboo】
People who are allergic to rifampicin, any of its components, or rifamycins are prohibited. Rifampicin is contraindicated in patients who have been treated with ritonavir and saquinavir, because it may increase the risk of severe liver toxicity. Rifampicin is contraindicated in patients who have been treated with atazanavir, darunavir, fosamprenavir, saquinavir or tiranavir, because rifampicin can potentially greatly reduce the risk of these antiviral drugs. Plasma concentration, which may result in the loss of antiviral activity and/or the development of viral resistance.
【Precautions】
caveat
Rifampicin has been shown to cause liver dysfunction. Patients with liver disease who took rifampicin and other hepatotoxic drugs at the same time had cases of death from jaundice. Patients with impaired liver function should be given rifampicin only when necessary and should be used with caution and strict medical supervision.
These patients should be closely monitored for liver function, especially ALT and AST should be monitored every 2-4 weeks before and during treatment. If there are signs of liver damage, rifampicin should be discontinued.
In some cases, hyperbilirubinemia at the beginning of treatment can cause competition between rifampicin and bilirubin at the cellular level in the liver's excretion pathway. A single report showed that the level of bilirubin and/or transaminase increased moderately, which is not an indicator of interruption of treatment in itself; on the contrary, after repeating the test, pay attention to the trend of indicators and make a decision based on the patient's clinical condition.
Rifampicin has enzyme-inducing properties, including the induction of Delta-aminolevulinic acid synthase.
Because meningococci may become resistant quickly, rifampicin is limited to short-term treatment of asymptomatic carriers. Rifampicin cannot be used for the treatment of meningococcal disease.
Precautions:
1. This product is only used for intravenous drip, not intramuscular injection or subcutaneous injection. Avoid liquid extravasation during infusion.
2. The infusion should be prepared for immediate use, and the preparation of the pharmaceutical solution can only be used once.
3. It cannot be mixed with other drugs to avoid precipitation. Combined with other intravenous drugs for treatment, it needs to be injected through different parts.
4. Rifampicin may rapidly develop bacterial resistance when used alone to treat tuberculosis, so this product must be combined with other anti-tuberculosis drugs.
5. Patients must be informed that rifampicin can turn urine, saliva, and tears into light red, and can permanently color contact lenses.
6. Use with caution in persons with alcoholism and liver damage.
7. This product is mainly used for hospitalized patients, especially for patients who cannot tolerate oral treatment, such as post-operative, comatose, and impaired gastrointestinal absorption.
8. Rifampicin can cause leukopenia and thrombocytopenia, and cause bleeding and infection of the gums, and delayed wound healing. At this time, surgery such as tooth extraction should be avoided, and oral hygiene should be paid attention to. Care should be taken when brushing and flossing teeth until the blood picture returns to normal.
9. Interference with diagnosis: it can cause direct antiglobulin test (Coombs test) to be positive; interfere with the determination of serum folic acid concentration and serum vitamin B12 concentration; it can cause retention of sulfonate sodium test, so sulfonate sodium test should be in Before daily intravenous infusion of rifampicin, to avoid false positive results, rifampicin can interfere with the results of various urinalysis tests performed by spectrophotometer or color change, because the use of rifampicin can make urine The liquid is orange-red or red-brown. Taking rifampicin can increase the measurement results of blood urea nitrogen, serum alkaline phosphatase, serum alanine aminotransferase, aspartate aminotransferase, serum bilirubin and serum uric acid concentration.
[Medicine for pregnant women and lactating women]
Teratogenic effects of pregnancy
Rifampicin has been shown to have teratogenic effects on rodents. During organ formation, the oral administration of rifampicin at a dose of 150 to 250 mg/kg/day (about 1 to 2 times the maximum recommended human dose calculated based on body surface area) caused congenital malformations in the offspring of pregnant mice, mainly in Spina bifida increased. When the oral dose of pregnant mice is 50 to 200 mg/kg (approximately 0.2 to 0.8 times the maximum recommended human dose calculated based on body surface area), rifampin causes a dose-dependent increase in fetal cleft palate. When oral administration of rifampicin to pregnant rabbits is as high as 200 mg/kg/day (about 3 times the maximum recommended human dose calculated based on body surface area), it can cause bone formation disorders and embryo toxicity. There is no adequate and strict controlled study of rifampicin in pregnant women. Rifampicin has been reported to cross the placental barrier and appear in the cord blood. Rifampicin can only be used during pregnancy when the potential benefits to the fetus are greater than the potential risks.
Pregnancy-non-teratogenic effects
In the last few weeks of pregnancy, administration of rifampicin can cause postpartum hemorrhage in women and infants, and vitamin K treatment can be given appropriately.
Breastfeeding women
Since rifampicin has been found to have potential carcinogenic effects in animal experiments, it is considered that stopping breastfeeding or drug withdrawal depends on the importance of drug treatment to the mother.
【Children's Medication】
The recommended dose for children with tuberculosis is 10-20mg/kg, once a day, and the dose should not exceed 600mg/day;
Pharmacokinetic characteristics of children's medication: In a study on children aged 0.25 to 12.8 (n=12) using this product, it was found that the peak blood concentration of rifampicin was about 25.9±1.3µɡ/ml; 1 to 4 days after the initial treatment, the peak blood concentration ranged from 11.7 to 41.5µɡ/ml; 5 to 14 days after the initial treatment, the peak blood concentration ranged from 13.6 to 37.4µɡ/ml. The plasma half-life is 1.04 to 3.81 hours at the beginning of treatment, and 1.17 to 3.19 hours after 5 to 14 days of initial treatment.
【Geriatric Medication】
Because there are not enough clinical research data on the use of this product in patients aged 65 and above, the use of this product in elderly patients should be closely monitored.
【medicine interactions】
1. Rifampicin is an inducer of liver enzymes. When rifampicin is used in combination with drugs that undergo biotransformation through this metabolic pathway, it can accelerate the metabolism of these drugs. In order to obtain the best therapeutic effect, the dosage of these drugs needs to be adjusted when starting or stopping the combination of rifampin. It is reported that rifampicin can accelerate the metabolism of the following drugs: anticonvulsants (such as phenytoin), antiarrhythmic drugs (such as diisopyramide, mexiletine, quinidine, tocainide), oral anticoagulants , Antifungal drugs (such as fluconazole, itraconazole, ketoconazole, etc.), barbiturates, β-blockers, calcium ion blockers (such as diltiazem, nifedipine, vitamin Lapamil, etc.), chloramphenicol, clarithromycin, corticosteroids, cyclosporine, digitalis glycosides, clofibrate, hormonal contraceptives, dapsone, diazepam, doxycycline, fluoroquinolones ( Such as ciprofloxacin), haloperidol, oral hypoglycemic drugs (sulfonylureas), levothyroxine, methadone, narcotic analgesics, nortriptyline, progesterone, quinine, theophylline, tacro Moss, tricyclic antidepressants (amitriptyline, nortriptyline), and azidothymidine. The dosage of these drugs should be adjusted accordingly when rifampicin is used in combination.
2. Rifampicin can promote the metabolism of estrogen or reduce its enterohepatic circulation, reduce the effect of oral contraceptives, and cause irregular menstruation, inter-menstrual bleeding and unplanned pregnancy. When patients use rifampin, they should switch to other contraceptives. method.
3. Rifampicin can induce liver microsomal enzymes, increase the metabolism of the antitumor drugs dacarbazine and cyclophosphamide, the formation of alkylated metabolites, and promote the reduction of white blood cells, so the dosage needs to be adjusted.
4. The combination of isoniazid with rifampicin or miconazole (intravenous) and ketoconazole can increase the risk of liver toxicity, especially in patients with original liver damage and fast isoniazid acetylation. In addition, the combination of isoniazid or rifampicin with miconazole or ketoconazole can reduce the plasma concentration of the latter two, so this product and isoniazid should not be combined with imidazoles.
5. The combination of rifampicin and diazepam (Valium) can increase the elimination of the latter and reduce the blood concentration, so the dose needs to be adjusted.
6. The combination of rifampicin and ethionamide can aggravate its adverse reactions.
7. Rifampicin can increase the degradation of levothyroxine in the liver, so the dose of levothyroxine should be increased when the two are used in combination. Rifampicin can also increase the metabolism of methadone and mexiletine in the liver, causing symptoms of methadone withdrawal and a decrease in the blood concentration of mexiletine. Therefore, the latter two need to be adjusted when used in combination.
8. Rifampicin can increase the metabolism of phenytoin sodium in the liver, so when the two are used in combination, the blood concentration of phenytoin sodium should be measured and the dosage should be adjusted.
9. The combination of probenecid and rifampicin can increase the blood concentration of rifampicin and produce toxic reactions.
【Overdose】
Symptoms and signs
Nausea, vomiting, abdominal pain, skin itching, headache, and drowsiness will occur soon after the medication. In severe liver disease, a coma may occur. There may be a temporary increase in transaminase and/or bilirubin. The skin, urine, sweat, saliva, tears, and feces can be brownish-red or orange-colored, and its intensity is proportional to the amount of medication.
The liver is enlarged and tender, and can progress within a few hours after a severe overdose; the bilirubin level rises, and the jaundice progresses rapidly. Other physical examination results remained basically normal. Less directly affect the hematopoietic system, electrolyte levels or acid-base balance.
Edema around the face or orbits of pediatric patients has also been reported. In some deaths, there have been reports of hypotension, sinus tachycardia, ventricular arrhythmia, seizures and cardiac arrest.
Acute toxicity
The minimum acute lethal or toxic dose has not been established. According to reports, adult non-fatal acute drug overdose, the dose range is 9-12g; adult fatal acute drug overdose, the dose range is 14-60g, these cases include some alcoholics or people with a history of alcohol abuse; 1-4 years old The non-lethal overdose for pediatric patients is 100 mg/kg, one or two doses.
deal with
(1) Develop personalized and strengthened support measures.
(2) Fix the airway and establish sufficient breathing exchange.
(3) Gastric lavage, because patients often have nausea and vomiting, it is not appropriate to induce vomiting; after gastric lavage, activated charcoal paste is given to absorb the residual rifampicin in the gastrointestinal tract; antiemetics are given to patients with severe nausea and vomiting.
(4) Give diuretics to promote drug excretion.
(5) In severe cases, hemodialysis or peritoneal dialysis can be performed.
【Pharmacology and Toxicology】
Pharmacological action
Rifampicin inhibits the DNA-dependent RNA polymerase activity of sensitive bacteria and specifically affects the RNA polymerase activity of bacteria without affecting mammalian cells. It has been confirmed that the therapeutic dose of rifampicin has killing activity on both intracellular and extracellular Mycobacterium tuberculosis. The resistance of bacteria to rifampicin is similar to other rifamycins. Rifampicin has a killing effect on slow-growing and quiescent tubercle bacilli. It also has significant bactericidal activity against isolated Neisseria meningitidis.
In the treatment of tuberculosis and meningococcal carriers, a small number of drug-resistant bacteria that emerge at any time are the key to treatment. In addition, the resistance of bacteria to rifampicin has been determined to be caused by the DNA-dependent RNA polymerase undergoing a one-step mutation. Due to the rapid development of drug resistance, if there is continuous positive culture during the treatment process, a drug susceptibility test should be done.
Rifampicin has antibacterial activity against most strains of the following microorganisms: (1) Aerobic Gram-negative bacteria; Neisseria meningitidis; (2) Other bacteria; Mycobacterium tuberculosis.
Rifampicin has an in vitro antibacterial effect on most of the following bacterial strains, but its safety and effectiveness in the treatment of infectious diseases caused by the following microorganisms have not been confirmed by fully and strictly controlled clinical studies; (1) Aerobic Gram-positive bacteria; Staphylococcus (including methicillin-resistant Staphylococcus), Staphylococcus epidermidis; (2) Aerobic Gram-positive bacteria; Haemophilus influenzae (3) Other bacteria; Mycobacterium leprosy .
β-lactamase has no effect on the activity of rifampicin.
Toxicology Research
Genotoxicity: In the study of bacteria, fruit flies or mice, this product has not been found to have mutagenic effects; however, when the whole blood cell culture of this product was carried out, it was found that chromosome breakage increased. In patients treated with the combination of streptomycin, rifampicin, isoniazid and pyrazinamide or the combination of rifampicin, isoniazid, and pyrazinamide, an increased rate of lymphocyte chromosomal abnormalities was found.
Reproductive toxicity: When oral administration of rifampin 15 to 25 times the human dose to rodents, it produces teratogenic effects. It is reported that rifampicin can pass through the placental barrier, but clinically, rifampicin for injection is used alone or in combination with other anti-tuberculosis drugs, and there are not enough studies to confirm the effect on the fetus. Although there are individual teratogenic reports in the literature, but There has not been a fully and strictly controlled study in pregnant women. Oral Rifampicin 150~250mg/kg has teratogenic effects on mice and rats, mainly causing cleft palate in mice and spina bifida in rats, and is related to the dose, which is as high as 20 times the human dose in pregnant rabbits. Rifampicin can cause bone formation disorders and embryo toxicity.
Since rifampicin has been found to have potential carcinogenic effects in animal experiments, it is considered that stopping breastfeeding or drug withdrawal depends on the importance of drug treatment to the mother.
Carcinogenicity: There is no report about the potential carcinogenic, mutagenic and reproductive toxicity of this product for long-term use. There are clinical reports of several patients with accelerated lung cancer growth after taking this product, but its relevance to the drug has not been determined. The mice were given 2 to 10 times the average daily dose of humans for 60 weeks. At the 46th week, female mice (a type of mice susceptible to liver cancer) were observed to develop related liver cancer. However, rifampicin has not been found to have carcinogenic effects in similar tests on male mice of the same species mentioned above, as well as other species of mice or rats.
A number of studies have suggested that rifampicin has potential immunosuppressive effects on rabbits, rats, mice, guinea pigs and human lymphocytes in vitro, but in vitro studies have also shown that rifampicin has anti-tumor activity.
【Pharmacokinetics】
According to literature data: healthy male volunteers received 300 or 600 mg of rifampicin intravenously, the peak blood concentrations were 9.0±3.0 and 17.5±5.0 µɡ/ml, and the plasma clearance rates were 0.19±0.06 and 0.14±0.03L/hr/ ㎏, the steady-state volume of distribution is 0.66±0.14 and 0.64±0.11L/㎏, respectively. The plasma half-life of oral rifampicin is about 3 hours. After intravenous infusion of 300 or 600 mg rifampicin, its plasma concentration can still be detected at 8 and 12 hours after administration. Intravenous infusion of 600 mg rifampicin, its plasma concentration did not increase proportionally to 300 mg administration, but increased by 30% from the theoretical value, indicating that high-dose administration was slowed down, continuous intravenous infusion of 600 mg rifampicin, plasma concentration From 5.81±3.38µɡ/ml at 8 hours after administration on the first day to 2.6±1.88µɡ/ml at 8 hours after administration on the 7th day.
Rifampicin is widely distributed in the body, reaching effective concentration levels in many organs and body fluids (including cerebrospinal fluid). The plasma protein binding rate is 80%. The unbound part diffuses freely into the tissue.
Rifampicin is mainly metabolized in the liver, excreted in the bile, then enters the enterohepatic circulation, and is deacetylated to produce 25-desacetyl rifampicin. This main metabolite still has a certain antibacterial activity. After deacetylation, the reabsorption of the small intestine is reduced, facilitating the excretion of rifampicin. About 30% of the drug is excreted in the urine, half of which is the original drug.
[Storage] Sealed and stored in a cool, dark (no more than 20°C) dry place.
[Packing] Low borosilicate glass control injection bottle, 1 bottle/box
[Validity] 24 months
[Executive standard] Implement drug registration standard YBH01932015 and meet the requirements of the 2015 edition of the Chinese Pharmacopoeia
[Approval Number] National Medicine Standard H20153165
【manufacturer】
Company Name: Ruiyang Pharmaceutical Co., Ltd.
Production address: No. 1, Ruiyang Road, Yiyuan County, Shandong Province
Postal Code: 256100
Phone number: 4006 123458; 15853312365
Fax number: 0533-3248777
Website: http://www.reyoung.cn
Mailbox: reyoung@reyoung.cn
Scan the QR code to read on your phone
Scan the QR code to read on your phone
Previous
Rifampicin Capsules(0.15g)
相关产品


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263