Number of views:
1000
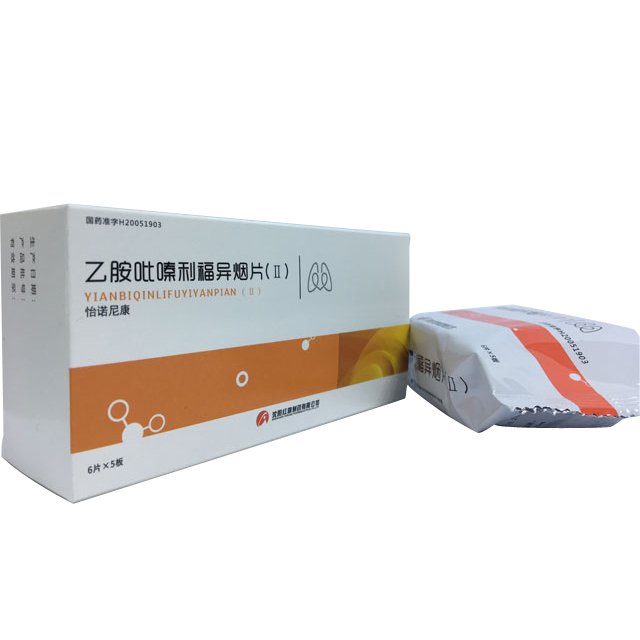
Ethylpyrazine Rifamil Tablets (Ⅱ)
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Quantity
-
+
Stock:
Category
1
Product description
Parameters
Ethidium pyrazine Rifasity smoke tablets (Ⅱ) instructions
Please read the instructions carefully and use under the guidance of a doctor
【Drug Name】
Generic name: Ethidium pyrazine and Rifisoyan tablets (Ⅱ)
Commodity name: Yinuo Nikon
English name: Ethambutol Hydrochloride, Pyrazinamide, Rifampicin and Isoniazid Tablets (Ⅱ)
Chinese Pinyin: Yi'an Biqin Lifu Yiyan Pian (Ⅱ)
【Ingredients】
This product is a compound preparation, and its components are: each tablet contains rifampicin (C 43 H 58 N 4 O 12 ) 0.15 g, isoniazid (C6H7N3O) 0.075 g, pyrazinamide (C 5 H 5 N 3 O) ) 0.4 g, ethambutol hydrochloride (C 10 H 24 N 2 O 2 ·2HCl) 0.275 g.
【Properties】
This product is a film-coated tablet, which shows orange-red to dark red after removing the coating.
【Indications】
It is suitable for the first 2 months of intensive treatment of tuberculosis short-term therapy, and must be taken daily at this stage.
【specification】
Each tablet contains 150mg of rifampicin, 75mg of isoniazid, 400mg of pyrazinamide and 275mg of ethambutol hydrochloride
【Dosage】
oral. Patients weighing 30-37kg take 2 tablets daily, patients weighing 38-54kg 3 tablets daily, patients weighing 55-70kg 4 tablets daily, and patients weighing more than 71 kg take 5 tablets daily, one hour before meals. This product is not suitable for patients weighing less than 30kg.
This product is used in the intensive treatment phase of short-term anti-tuberculosis therapy, that is, the first 2 months of the treatment. According to WHO recommendations, continue treatment with rifampicin and isoniazid for at least 4 months after this stage.
If the initial intensive treatment with this product is interrupted, the reasons include the patient's unwillingness to take the drug or the occurrence of contraindications, continue the treatment, rifampicin, isoniazid, pyrazinamide, ethambutol hydrochloride must be taken alone, because rifampicin needs to be taken with Take the lower dose again.
Or follow the doctor's advice.
【Adverse reactions】
According to the literature report as follows:
Frequency: Common adverse reactions: >1/100
Uncommon adverse reactions: ≥1/1,000 and ≤1/100
Rare adverse reactions: ≥1/10,000 and ≤1/1,000
Very rare adverse reactions: <1/10,000
The adverse reactions during continuous and intermittent treatment of rifampicin are as follows
Blood and lymphatic system disorders are rare: temporary leukopenia, eosinophilia. In a few cases, intermittent treatment is more prone to thrombocytopenia and thrombocytopenic purpura symptoms than continuous treatment. There are reports that if you continue to take rifampicin after purpura appears, cerebral hemorrhage will occur and death will occur. Hemolysis and hemolytic anemia.
Endocrine disorders rare: menstrual disorders (amenorrhea rare cases); caused Addison's disease patients crisis.
Insane insanity.
Nervous system disorders are common: fatigue, drowsiness, headache, mild headache, dizziness.
Rare: Ataxia, muscle weakness.
Eye abnormalities are common: jealous, and can cause permanent discoloration of contact lenses.
Rare: Visual impairment. Severe symptoms, such as exudative conjunctivitis.
Gastrointestinal abnormalities are common: anorexia, nausea, abdominal pain, and bloating.
Rare: vomiting, diarrhea, erosive gastritis and pseudomembranous colitis.
Skin and subcutaneous tissue abnormalities are common: flushing, itching, rash, urticaria.
Rare: severe systemic allergic reactions, such as exfoliative dermatitis, Lyell's syndrome, pemphigoid.
Hepatobiliary abnormalities are common: asymptomatic increase in liver drug enzymes.
Rare: hepatitis, jaundice, induced porphyria.
Kidney and urinary system abnormalities are rare: blood urea nitrogen and uric acid content increase, acute renal failure causes hemoglobinuria, hematuria and chronic interstitial nephritis, glomerulonephritis, and renal tubular necrosis.
General adverse conditions are common: body fluids and secretions such as urine, saliva, tears, feces, sputum and sweat may be orange-red.
Rare: collapse, shock, edema.
Adverse reactions of intermittent rifampicin therapy or resumption of treatment after stopping the drug
When the patient uses rifampin, it is not the daily routine medication or the resumption of treatment after the temporary interruption of the administration. Common "flu-like syndrome" occurs, which may be caused by immunopathological reactions. Manifested as fever, tremor, headache, dizziness and muscle pain. Occasionally, thrombocytopenia, purpura, dyspnea, asthma, hemolytic anemia, shock and acute renal failure may occur. When rifampicin is temporarily discontinued and treatment is resumed or rifampicin is given a weekly dose (≥25mg/kg), this severe syndrome may occur suddenly and not appear in the progressive "flu-like syndrome" .
Adverse reactions of isoniazid
Blood and lymphatic system abnormalities are rare: eosinophilia, thrombocytopenia, anemia (hemolytic anemia, iron deficiency anemia).
Very rare: agranulocytosis.
Endocrine abnormalities are rare: interfere with the liver metabolism of individual hormones, cause menstrual disorders, gynecomastia, Cushing’s syndrome, uncontrollable diabetes, hyperglycemia, metabolic acidosis.
Mental disorders are rare: mental disorders, excessive excitement, euphoria, and insomnia.
Nervous system abnormalities are common: peripheral neuritis (common in patients with large doses, malnutrition, alcoholism, slow acetylation, and diabetes) manifests as numbness in the hands and feet, and a tingling sensation.
Rare: optic nerve damage, convulsions, dizziness, headache, viral encephalitis, large doses can increase the frequency of seizures in patients with epilepsy.
Gastrointestinal abnormalities are common: nausea, vomiting, upper abdominal pain.
Hepatobiliary abnormalities are common: liver dysfunction (usually a mild or transient increase in serum transaminase levels), the common prodromal symptoms are poor appetite, nausea, vomiting, fatigue, depression, and weakness.
Rare: hepatitis, severe hepatitis.
General adverse conditions are common: allergies, drug rash, fever.
Rare: allergies, dry mouth, heartburn, frequent urination, rheumatism, systemic lupus erythematosus, pellagra, vasculitis, lymph node disease, acne.
Adverse reactions of pyrazinamide
Blood and lymphatic system abnormalities are rare: thrombocytopenia, iron deficiency anemia, coagulopathy, splenomegaly.
Gastrointestinal abnormalities are common: nausea, vomiting, anorexia, abdominal pain.
Hepatobiliary abnormalities are common: moderate or transient increase in serum transaminases during early treatment, porphyria.
Rare: Severe liver poisoning, hepatomegaly, and jaundice related to the dose.
Kidney and urinary system abnormalities are common: hyperuricemia (usually asymptomatic), gout.
Rare: chronic interstitial nephritis, urinary retention.
General adverse conditions are common: allergies, mild joint pain, muscle pain.
Rare: allergies, rash, photosensitivity, urticaria, itching, fever, acne.
Adverse effects of ethambutol
Blood and lymphatic system abnormalities are rare: thrombocytopenia, leukopenia.
Mental abnormalities are not common: hallucinations.
Nervous system abnormalities are not common: dizziness, disorientation, confusion, headache, malaise.
Rare: peripheral neuritis (numbness, tingling, burning, weakness in hands and feet).
Eye abnormalities are rare: dose-dependent posterior optic neuritis (blurred vision, eye pain, red-green color blindness, blindness).
Gastrointestinal abnormalities are uncommon: abdominal pain, loss of appetite, nausea and vomiting, anorexia.
Skin and subcutaneous tissue abnormalities Uncommon: itching, urticaria, rash.
Abnormalities in the kidneys and urinary system. Uncommon: elevated blood uric acid can cause acute attacks of gout (chills, joint pain, swelling, especially thumbs, ankles and knees).
General adverse symptoms are rare: hypersensitivity reactions (rash, fever, joint pain), allergic reactions.
【Taboo】
1. People who are allergic to rifampicin, pyrazinamide, isoniazid, ethambutol hydrochloride or any excipients are prohibited;
2. Patients with abnormal liver function, patients with biliary obstruction, pregnant women within 3 months, patients with gout, patients with mental illness, epilepsy, patients with diabetic fundus disease, and porphyria are disabled;
3. Patients with severe renal insufficiency (creatinine clearance rate <30ml/min) are forbidden;
4. It is contraindicated to use in combination with voriconazole and protease inhibitors (see [Drug Interactions] for details).
【Precautions】
caveat
Due to the existence of acetylation subtypes in the population, patients with extremely fast or extremely slow acetylation should take four drugs separately, so that the dose of isoniazid can be easily adjusted.
If severe acute allergic reactions occur while using this product, stop immediately, such as: thrombocytopenia, purpura, hemolytic anemia, dyspnea and symptoms similar to asthma, shock or renal failure, etc., in the treatment of rifampicin very few These adverse reactions may also occur in some cases, and the patient should no longer take rifampicin when the above-mentioned adverse reactions occur.
It should also be stopped when other allergic symptoms appear, such as fever or skin reactions. For safety reasons, rifampicin should not be used or resumed during treatment.
When using this product to treat patients with vision defects, more attention should be paid. It is recommended that eye examinations should be done regularly during the start and use process (especially when using higher doses), including examinations of resolution, color discrimination and visual field. The visual inspection performed by each patient during the treatment should be recorded, and the patient should be advised to stop continuing to use it if there is a visual disease during the clinical treatment.
Precautions
The precautions of this product are the same as those of rifampicin, isoniazid, ethambutol and pyrazinamide.
It is recommended that patients should be treated without interruption.
Damage to liver function, malnutrition, alcoholism
Rifampicin, isoniazid, ethambutol, and pyrazinamide are mainly metabolized in the liver, so transaminase usually rises above the upper limit of the normal range (ULN) during the administration. There may be liver dysfunction in the first few weeks of treatment, but there is no need to interrupt the treatment, and it will naturally return to the normal range in the third month of treatment.
While using rifampicin, although mild transaminase elevations often occur, jaundice or hepatitis is rare. In patients taking isoniazid and rifampicin at the same time, the increase in alkaline phosphatase is mainly caused by rifampicin, while the increase in transaminase may be caused by isoniazid, rifampicin, and pyrazinamide respectively, or It is caused by the combined action of the three.
Patients with liver damage should be used with caution during treatment, and drug monitoring should be strictly carried out. In these patients, careful liver function monitoring should be performed, especially when serum alanine aminotransferase (SGPT/ALAT) and serum aspartate aminotransferase (SGOT/ASAT) are present. Liver function monitoring should be performed before and every week or every two weeks during treatment. . If symptoms of liver cell damage occur, the drug should be discontinued immediately.
If the bilirubin and/or transaminase is moderately elevated, it is not necessary to stop the treatment; but after repeated tests of liver function, the change trend of bilirubin and/or transaminase should be judged in connection with the patient's clinical physical condition.
When a patient develops jaundice or transaminase exceeds 3 times the upper limit of normal range (ULN), it is recommended to stop using isoniazid. In order to facilitate the treatment of this clinical situation, the compound drug should be replaced with a single component of rifampicin, isoniazid, pyrazinamide or ethambutol.
If liver function fails to return to normal or transaminase exceeds 5 times the upper limit of the normal range, rifampicin, pyrazinamide, and ethambutol should be discontinued. In order to facilitate treatment, the fixed-dose compound preparation should be replaced with a single-component pharmaceutical preparation.
When using isoniazid, patients with chronic liver disease should be closely monitored. Isoniazid can cause severe and even fatal hepatitis, which may occur within months of its treatment. Although hepatotoxicity caused by the use of isoniazid is rare in patients around the age of 20, its frequency increases with age, and the probability of occurrence in patients over the age of 50 reaches 3%. Strict liver function monitoring will reduce the incidence of hepatotoxic reactions. Patients should be monitored for precursor symptoms related to hepatitis, such as fatigue, weakness, malaise, anorexia, nausea, and vomiting. Once these symptoms appear, or liver damage is found, drug treatment should be stopped immediately. If you continue to use this product for treatment, it may aggravate the degree of liver damage.
Patients with chronic liver disease are the same as patients with chronic alcoholism and malnutrition. When using this product to treat tuberculosis, the damage to the liver should be reduced as much as possible. If anti-tuberculosis treatment is necessary, then the dose of rifampicin, isoniazid, pyrazinamide, ethambutol should be adjusted, and this product is not suitable for such patients.
Because taking high doses of isoniazid can cause malnutrition or insufficient vitamin B6 production in elderly patients, vitamin B6 supplementation may be necessary.
Impairs kidney function
Patients with severe renal insufficiency, slow elimination of isoniazid, pyrazinamide, ethambutol, increase the drug concentration in the body, and increase adverse reactions. Patients with moderate renal impairment (creatinine clearance rate 30-60ml/min ) This product should be used with caution.
gout
Patients with a history of gout should use pyrazinamide and ethambutol with caution. Check blood uric acid regularly during taking. The drug should be discontinued when the patient has gouty arthritis.
Blood disease
When prolonging the duration of treatment and treating patients with liver dysfunction, a complete blood cell test should be done. Once purpura or thrombocytopenia occurs, rifampicin should be stopped immediately. Pyrazinamide may affect clotting time or vascular integrity, and patients with symptoms of hemoptysis should be informed.
diabetes
It has been reported that diabetic patients taking isoniazid will increase the difficulty of controlling diabetes.
epilepsy
Because isoniazid and ethambutol have neurotoxic effects, they should be closely observed when treating patients with a history of convulsions.
Neuropathy
Patients with peripheral or optic neuritis should be used with caution. When a patient has a history of alcohol abuse, neurological examinations should be done regularly. Vitamin B6 supplementation can delay or reduce the damage of isoniazid to the nervous system of patients, especially the elderly and malnourished patients. The dosage of vitamin B6 should be administered under the guidance of a licensed doctor.
contraception
During treatment with rifampicin, another non-hormonal method of contraception should be used.
alcohol
During the treatment with this product, the patient should abstain from alcohol.
Laboratory examination
Before and during treatment, complete blood count examination, liver function examination (SGPT/ALAT, SGOT/ASAT), renal function examination and uric acid monitoring should be done regularly. When applying ethambutol treatment, it is recommended to do a vision check.
Combination therapy
Rifampicin is an effective inducer of the cytochrome P450 system, which can promote the drug metabolism of co-administered drugs, reduce the blood concentration and reduce the efficacy of the drug. If you have the ability to monitor the metabolism of the drug in the liver and adjust the dose, the drug can still be used in combination with rifampicin.
This product is not suitable for combined use with the following drugs: nevirapine, simvastatin, oral contraceptives and ritonavir (when the drug is used in low doses, the blood concentration will be significantly reduced).
This product will slightly affect the driving ability and the ability to use the machine. The adverse reactions caused by ethambutol such as confusion, disorientation, hallucinations, dizziness, discomfort and visual disturbance may affect the ability of the drug user to drive and use machinery.
[Medicine for pregnant women and lactating women]
It is contraindicated in pregnant women in the first 3 months of pregnancy.
If the medicine may be beneficial to the patient, the treatment plan should be considered. This product should only be used when the potential benefit to the mother is greater than the potential risk to the fetus.
Rifampin
The limited published clinical data on use during pregnancy shows that it does not increase the possibility of fetal teratogenesis. Rifampicin can pass through the placenta. The use of rifampicin in the last few weeks of pregnancy can cause postpartum hemorrhage in the mother and newborn. Animal studies have shown that when the dose is ≥150mg/kg, reproductive toxicity will occur.
Isoniazid
According to limited data, this product did not show the expected congenital teratogenicity. It can penetrate the placenta and produce neurotoxic effects on young children. Animal studies have shown that it has reproductive toxicity.
Pyrazinamide
There is no animal reproductive toxicity study of pyrazinamide. Whether pregnant women take pyrazinamide has harm to the fetus has not been confirmed.
Ethambutol
It can penetrate the placenta and may cause the fetus's blood concentration to be 30% of the mother's. Limited clinical data show that it will not increase the possibility of fetal teratogenesis in the human body, and animal studies have shown that there is a potential teratogenic risk.
It is recommended to take vitamin K orally in the mother and newborns after delivery in the last month of pregnancy, because rifampicin can cause bleeding in the mother and newborn.
Because isoniazid may have neurotoxic effects on young children, vitamin B6 supplementation is required during pregnancy.
Rifampicin, isoniazid, pyrazinamide and ethambutol can pass into breast milk, but adverse reactions to nursing infants have not been observed. However, in view of the theoretical neurotoxic effects of isoniazid and ethambutol, breastfeeding is not recommended during the medication.
【Children's Medication】
This product should not be used for children.
【Geriatric Medication】
The elderly are often accompanied by physiological decline in renal function, so the dosage should be adjusted according to renal function. Or follow the doctor's advice.
【medicine interactions】
The effect of other drugs on this product
Antacids can reduce the bioavailability of rifampicin, isoniazid and ethambutol. To avoid this, this product should be taken at least 1 hour before taking the antacid. Corticosteroids can reduce the plasma concentration of isoniazid by promoting the metabolism of isoniazid and/or increasing renal clearance.
The effect of this product on other drugs
Rifampicin has a strong cytochrome P450 system (CYP450) inducing effect. The cytochrome P450 system is mainly composed of two sub-families, CYP3A and CYP2C, accounting for 80% of the isoenzymes of the cytochrome P450 system. When combined medications, rifampicin can accelerate the metabolism of combined medications. Part or all of these drugs taken at the same time are metabolized by these two isoenzymes. Moreover, rifampicin can also induce uridine-5'-diphosphate-glucuronidyl transferase (it is another enzyme of drug metabolism), which will cause the blood concentration of other drugs applied at the same time to be lower than the therapeutic dose The blood concentration of the drug increases or decreases the effect of side effects. Isoniazid can inhibit the metabolism of these drugs and increase their blood drug concentration.
Moreover, rifampicin and isoniazid can affect the antagonism of some drugs, such as phenytoin, warfarin and theophylline. The net effect is unpredictable and may change over time.
Drugs that are mainly eliminated by liver metabolism can still be used in combination with this product if the blood concentration or adverse reactions can be monitored, and the dosage can be adjusted appropriately. Check regularly during the period of taking this product and after stopping treatment for 2 to 3 weeks.
The effect of rifampicin reaches its peak within ten days under enzyme induction, and gradually decreases two weeks after the drug is stopped. If the dosage of other drugs is increased during the use of this product, these factors need to be considered when stopping.
If you want to consider whether this product has an effect on the concentration of compatible drugs, you can refer to the following:
Interactions of rifampicin
It is strictly forbidden to use this product at the same time with the following drugs: voriconazole and protease inhibitors, except when ritonavir is in sufficient dosage or 600mg twice daily.
This product should not be used at the same time with the following drugs: nevirapine, simvastatin, oral contraceptives and ritonavir (when low-dose medication, the blood concentration will be significantly reduced).
When this product is used simultaneously with the following drugs, some specific parameters need to be monitored or clinically monitored, such as: calcium antagonists, type Ia antiarrhythmic drugs (quinidine, dasulpine), oral anticoagulants, Pyrrole antifungal drugs (except voriconazole), buspirone, carvedilol (due to its low therapeutic window for myocardial insufficiency and the symptom), immunosuppressants (such as cyclosporine, tacrolimus, Sirolimus), clozapine, corticosteroids, gestrinone, estrogen and combined progesterone replacement therapy, haloperidol, thyroid hormones, methadone, morphine, efavirenz, propafenone, terbina Fen, Tiagabine, Zidovudine, Zolpidem, Zaleplon, Carbamazepine, Phenytoin, Theophylline, Diazepam, Digitalis, Dapsone, Atovaquone, Repaglinide and Sulfonate Urea oral diabetes drugs, β-receptor antagonists (such as metoprolol, propranolol, which is metabolized by the liver), chloramphenicol, clarithromycin, telithromycin, tricyclic antidepressants, para-amino water Salicylic acid, cimetidine, mexiletine, nevirapine, fluvastatin, rofecoxib, imidapril, topxolone.
Interaction of Isoniazid
When this product is used with the following drugs, some specific parameters need to be monitored or clinically monitored, such as: anesthetics, glucocorticoids, ketoconazole, phenytoin, pyrazinamide, stavudine, carbamazepine, diazepam Pan, ethosuximide, theophylline.
Pyrazinamide interaction
When this product is used with the following medicines, some specific parameters need to be monitored or clinically monitored, such as probenecid, benzalkonium ketone.
Rifampicin can reduce the effect of oral contraceptives. Patients treated with this product should use non-hormonal contraception. Simultaneous use of antibiotics may inactivate oral typhoid vaccine.
Avoid eating foods rich in tyrosine and histidine. Isoniazid may inhibit monoamine oxidase and diamine oxidase. Eating foods containing tyrosine (such as cheese, red wine) or histidine (such as tuna) may cause headaches, palpitations, flushing and other symptoms.
During the gallbladder radiography, rifampicin may delay the secretion of bile.
During the use of rifampicin, microbiological methods cannot be used to determine the blood concentration of folic acid and cyanocobalamin (vitamin B12), because rifampicin will compete with bilirubin and BSP. To avoid false positive reactions, a BSP test should be performed the morning before taking rifampicin.
【Overdose】
Rifampin
Poisoning: Infants (1 to 4 years old) have typical skin symptoms after oral administration of 100 mg/kg rifampicin; adults take 15 g for severe poisoning, 12 g for moderate poisoning, and 60 g for extremely severe poisoning.
Symptoms: gastrointestinal discomfort, vomiting, night sweats, dyspnea, epilepsy, renal failure, liver failure, confusion, general itching, orange-red skin and urine, facial edema, lung swelling.
Treatment: gastric lavage, activated carbon is given after gastric lavage to absorb the residual rifampicin in the gastrointestinal tract; symptomatic treatment; renal failure requires dialysis.
Isoniazid
Poisoning: Alcohol can induce toxic reactions. The lethal dose for infants and young children is 80-150mg/kg; 15-year-old teenagers take 5 g for fatal poisoning; 8-year-old children take 900 mg for moderate poisoning; 3-year-old children take 2 to 3 g for severe poisoning; 15-year-old teenagers take 3 g, adults Taking 5 to 7.5g can cause extremely serious poisoning.
Symptoms: Typical symptoms are epilepsy and metabolic acidosis, urinary ketones, hyperglycemia; orbital myoclonus, dizziness, tinnitus, tremor, hyperreflexia, confusion, hallucinations; respiratory depression, asphyxia; tachycardia, arrhythmia, hypertension ; Nausea and vomiting, fever, rhabdomyolysis, diffuse intravascular coagulation, hyperglycemia, hyperkalemia, liver damage. The dose of isoniazid exceeding 10mg/kg can affect the peripheral nervous system and impair the patient's ability to drive or operate machinery.
Treatment: Patients with no history of epileptic seizures were given gastric lavage. After gastric lavage, activated carbon was given to adsorb residual isoniazid. The blood samples collected should be immediately tested for blood gas, ion, blood urea nitrogen, and blood sugar. When epileptic seizures and metabolic acidosis occur, 61 grams of vitamin B per gram of isoniazid; when the dose of seizures and isoniazid is uncertain, use 5 grams of vitamin B6 intravenously; no symptoms of epilepsy, prevent vitamin B B6 2-3 grams, intravenous injection. In order to reduce vascular irritation, vitamin B6 is diluted before use, and the drug is injected into the body with an infusion pump or syringe pump within 30 minutes, and vitamin B6 is repeatedly administered if necessary. Diazepam can enhance the effect of vitamin B6, and high-dose diazepam can be used alone to treat seizures. Severe cases have respiratory depression, prompt rescue; correct metabolic acidosis and ion disorders; diuresis, hemodialysis for severe poisoning; symptomatic treatment.
Pyrazinamide
Abnormal liver function, hyperuricemia.
Ethambutol
Loss of appetite, gastrointestinal disorders, fever, headache, dizziness, confusion, hallucinations.
【Pharmacology and Toxicology】
Pharmacological effects:
Rifampicin is a fungicide of the rifamycin class; isoniazid, pyrazinamide and ethambutol are anti-tuberculosis fungicides.
Mechanism
Rifampicin: In vivo and in vitro tests have shown that this product has obvious bactericidal effect on Mycobacterium tuberculosis and other atypical Mycobacterium tuberculosis; it has bactericidal effect on the inside and outside of the cells of microorganisms; this product inhibits the DNA dependence of sensitive strains Sex RNA polymerase, but does not affect the enzyme system of the host cell.
Isoniazid: It has a strong bactericidal effect on rapidly multiplying Mycobacterium tuberculosis. Its mechanism of action may be mainly to inhibit the synthesis of mycolic acid, which is an important part of the cell wall of mycobacteria.
Pyrazinamide: The exact mechanism of action is not very clear. Studies in vivo and in vitro have proved that pyrazinamide has antibacterial and bactericidal effects only under slightly acidic (PH 5.5) conditions.
Ethambutol: The mechanism of action has not been fully elucidated. This product can penetrate into mycobacteria and interfere with the synthesis of RNA, thereby inhibiting bacterial proliferation. This product is only effective for mycobacteria during the growth and reproduction period.
Microbial susceptibility
When the concentration of rifampicin in vitro is 0.005~0.2μg/ml, it inhibits the growth of mycobacteria; in vitro experiments show that this product can enhance the activity of streptomycin and isoniazid, but has no effect on the activity of ethambutol .
Isoniazid has a bacteriostatic effect on bacteria in the "quiescent phase" and bacteria in the "rapid proliferation phase"; the minimum concentration of anti-tuberculosis is 0.025~0.05μg/ml.
The MIC of pyrazinamide is 12.5-20μg/ml.
The MIC of various dosage forms of ethambutol is 0.5-2μg/ml. The antibacterial effect of ethambutol can be delayed for 24 hours, and the intensity of its antibacterial effect may be related to the contact time of the drug, rather than increasing the concentration of the drug.
Toxicological research:
Rifampin
Female mice taking high doses (2-10 times the clinical maximum dose) of rifampicin for 1 year, a significant increase in liver cancer cells in mice can be observed, and no carcinogenicity has been found in studies of other strains of mice and rats.
Studies have shown that this product is not mutagenic to bacteria and mice; blood cell culture with this product found that chromosomes are increased; there are research reports that this product has effects on rabbits, mice, rats, guinea pigs, lymphocytes in vitro and humans. Immunosuppressive effect.
The daily dose of rats, mice and rabbits during pregnancy exceeds 150mg/kg, and non-specific embryotoxicity can occur; the same dose can increase spinal division and cleft lip in rats and mice.
Isoniazid
This product has weak genetic toxicity. It produces pro-mutagenic agents during the formation of the toxic metabolites hydralazine and acetylhydralazine; after taking this product, no chromosomal changes are found in the lymphocytes of the patient, but it is used in combination. When the time, the frequency of chromosome changes increases. Whether this product can induce animal abnormalities is still controversial, but studies have proved that this product has embryo toxicity, and there is no report on its role in reproduction. A few data show that mice can cause lung cancer after being administered in a variety of ways, but there is evidence that the therapeutic and preventive doses of human anti-tuberculosis are not carcinogenic.
Pyrazinamide
This product has no carcinogenic effect on rats and male mice. In the Ames bacterial test, this product has no mutagenic effect, but it can induce chromosomal aberrations in human lymphocytes.
Ethambutol
Whether this product has genetic toxicity is still controversial (human lymphocyte culture test result is negative, rat cell nuclear test result is positive). Mice taking this product and sodium nitrite at the same time increase the incidence of lymphoma and lung cancer, but this product alone has no effect on the incidence of cancer.
In the study of reproductive toxicity in mice, after taking high doses of this product, animals may develop hare lips, brain malformations and spinal deformities; rats and rabbits after taking high doses of this product, occasionally cervical vertebra deformities and cyclops may occur in their offspring. , Short limb defects, cleft lip and harelip phenomenon.
【Pharmacokinetics】
Rifampin
Rifampicin is well absorbed on an empty stomach, and taking the medicine after eating can reduce the speed and degree of drug absorption. The plasma drug concentration reaches its peak at 2h, and is widely distributed throughout the body after absorption. In addition to meningitis, the drug concentration in the cerebrospinal fluid is low. The apparent volume of distribution of this product is about 55L, and the protein binding rate is as high as 80%. After the deacetylation reaction of this product, it becomes the active metabolite desacetyl rifampicin. This product and its metabolites are mainly excreted through bile, and this product has enterohepatic circulation. Approximately 10% of the prototype drug is excreted in urine.
The plasma elimination half-life of this product is 3 to 5 hours, and the half-life is shortened to 2 to 3 hours after multiple administrations. Due to its own liver drug enzyme induction, the elimination rate of rifampicin increased after 6-10 days of taking the drug. Taking this product in large doses, the excretion may be delayed due to the saturation of the biliary tract excretion.
Isoniazid
Oral absorption is fast, and the speed and degree of absorption of this product after eating are reduced. The blood drug concentration reached its peak after 1 to 2 hours. After absorption, isoniazid is widely distributed in the body fluids and tissues, and the apparent volume of distribution is about 43 L; the protein binding rate is low, only 0-10%. This product is acetylated into acetylisoniazid by N-acetyltransferase, and then bioconverted into isonicotinic acid and monoacetylhydrazine, which is hepatotoxic. The acetylation speed of this product is affected by genetic factors. People with slow acetylation often have a deficiency of N-acetyltransferase in the liver. About 50% of Caucasians and African Indians are of the slow metabolizing type; the vast majority of Eskimos and Asians such as Japan, China, and Vietnam belong to the fast metabolizing type.
Usually the half-life of this product is 1~4h, but due to the difference of acetylation speed, the range of change can be 0.5~6h. Nearly 75%-95% of isoniazid is excreted by the kidneys within 24 hours, most of which are the inactive metabolites N-acetylisoniazid and isoniazid.
Pyrazinamide
After oral administration, it is absorbed rapidly and completely in the gastrointestinal tract, and eating has no effect on absorption. The plasma concentration reaches its peak after 1 to 2 hours for adults, and the peak time for children is 3 hours. This product is hydrolyzed by microsomal deaminase into an active metabolite-pyrazinic acid, and then hydroxylated by xanthine oxidase to 5-hydroxylated pyrazinic acid. This product is mainly excreted through the kidneys in the form of metabolites, and only 3% is excreted in the form of prototype drugs through urine. The plasma half-life is approximately 10 hours.
Ethambutol
Oral absorption is good, the bioavailability is close to 80%, and eating does not affect its absorption. The blood drug concentration reaches its peak in 2 to 4 hours, and it is widely distributed in the body tissues and body fluids (except cerebrospinal fluid), but the drug concentration in the cerebrospinal fluid of patients with tuberculous meningitis can reach the therapeutic value. The drug concentration of red blood cells can be 2 to 3 times that of plasma drug concentration, the protein binding rate is low, only 20% to 30%, and the apparent volume of distribution is about 20L. This product is mainly metabolized by the liver, and about 15% is metabolized into inactive metabolites. The half-life is 3 to 4 hours, but the half-life of patients with renal insufficiency can reach 8 hours. About 80% (at least 50% of the prototype drug and nearly 15% of the inactive metabolites) are excreted by the kidneys within 24 h, and about 20% are excreted as prototypes in the feces.
Medications for special populations
Rifampin
In patients with renal insufficiency, 600 mg (10 mg/kg) per day can prolong the elimination half-life of the drug, leading to drug accumulation. Rifampicin cannot be eliminated by hemodialysis.
In patients with hepatic insufficiency, the plasma drug concentration increases and the elimination half-life is prolonged.
Isoniazid
Patients with slow acetylation metabolism and renal insufficiency may develop isoniazid drug accumulation. The blood concentration of isoniazid should be monitored and the dose should be reduced if necessary. In patients with liver dysfunction, the elimination half-life of isoniazid is prolonged.
Pyrazinamide
In patients with liver cirrhosis, the clearance rate of this product is significantly reduced, the half-life is prolonged, and the AUC of pyrazinic acid (the main metabolite) is increased by three times. The drug metabolism of this product for patients with renal insufficiency has not been reported, and this product can be cleared by hemodialysis.
Ethambutol
The elimination half-life of this product in patients with renal insufficiency is prolonged, and the dosage needs to be adjusted. This product cannot be cleared by hemodialysis.
【Storage】
Shade, seal, and store in a dry place.
【package】
(1) Aluminum-plastic blister packaging, 6 sheets/board; composite film packaging, 5 sheets/bag; carton packaging, 1 bag/box.
(2) Aluminum-plastic blister packaging, 6 sheets/board; composite film packaging, 5 sheets/bag; carton packaging, 2 bags/box.
(3) Aluminum-plastic blister packaging, 6 sheets/board; composite film packaging, 5 sheets/bag; carton packaging, 3 bags/box.
【Validity Period】
24 months.
【Executive Standard】
"Chinese Pharmacopoeia" 2015 Edition Two
【Approval Number】
National Medicine Standard H20051903
【manufacturer】
Company Name: Shenyang Hongqi Pharmaceutical Co., Ltd.
Scan the QR code to read on your phone
Scan the QR code to read on your phone
Previous
None
相关产品


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263