Number of views:
1000
Ethambutol Hydrochloride Capsules(0.25g)
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Quantity
-
+
Stock:
Category
1
Product description
Parameters
Ethambutol Hydrochloride Capsule Instructions
Please read the instructions carefully and use under the guidance of a doctor
【Drug Name】
Generic name: ethambutol hydrochloride capsules
English name: Ethambutol Hydrochloride Capsules
Chinese Pinyin: Yansuan Yi'andingchun Jiaonang
【Ingredients】
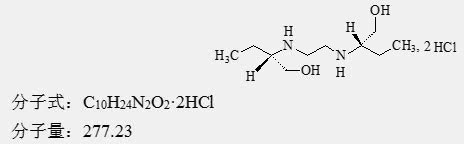
Chemical name: [2R,2[S-(R*, R*)]-R]-(+)2,2′-(1,2-ethylenediyldiimino)-bis-1-butanol Hydrochloride
Chemical Structure:
【Properties】
This product is a capsule, and the content is white crystalline powder.
【Indications】
This product is suitable for the treatment of tuberculosis and extrapulmonary tuberculosis caused by Mycobacterium tuberculosis in combination with other anti-tuberculosis drugs, and can also be used for the treatment of atypical Mycobacterium tuberculosis infection.
【specification】
0.25g
【Dosage】
Need to be used in combination with other anti-tuberculosis drugs.
1. Initial treatment: oral administration, 15mg/kg body weight, once a day; or 25-30mg/kg once, maximum 2.5g, 3 times a week; or body weight 50mg/kg, maximum 2.5g twice a week.
2. Retreatment: Orally, 25mg/kg body weight, once a day, after 60 days, followed by body weight 15mg/kg, once a day.
3. Atypical Mycobacterium tuberculosis infection: 15-25 mg/kg of body weight, once a day.
【Adverse reactions】
1. Common optic nerve damage, such as retrobulbar optic neuritis, optic nerve central fiber damage. It may be related to the decrease in the content of these metal elements after this product is chelated with copper, zinc and other metal elements. The incidence of retrobulbar optic neuritis is about 0.8%, which is related to the dose and course of treatment. Long-term medication and daily doses greater than 25mg/kg are prone to occur. The daily dose of 15mg/kg is 1%, 25mg/kg is 6%, 35mg /kg increased to 15%. Manifested as blurred vision, eye pain, red-green blindness or vision loss, and reduced vision. Early detection of the above reactions and timely discontinuation of the drug can disappear on their own within a few weeks or months, and permanent visual loss rarely occurs.
2. Rarely, chills, joint swelling and pain (especially the big toe, condyle, knee joint) and the skin on the surface of the diseased joint are heated and tight (acute gout, hyperuricemia).
3. Occasionally, gastrointestinal discomfort, nausea, vomiting, diarrhea, liver damage, peripheral neuritis (usually manifested as numbness, acupuncture sensation, burning pain or weakness of hands and feet) and allergic reactions (often manifested as skin rash, itching, headache, Fever, joint pain), etc.
【Taboo】
People who are allergic to this product, patients with known optic neuritis, alcohol poisoning, and those younger than 13 years old should use it with caution.
【Precautions】
1. Use with caution in patients with gout, optic neuritis, diabetic fundus disease, liver and kidney function decline. Patients with impaired renal function should reduce the dose.
2. Using this product alone, bacteria can quickly develop drug resistance, so it must be used in combination with other anti-tuberculosis drugs.
3. During treatment, check:
(1) Eyes, visual field, vision, red and green discrimination, etc., should be checked every month before and during the course of treatment, especially for patients with long treatment courses and daily doses of more than 15 mg/kg.
(2) Since this product can increase the blood uric acid concentration and cause gout attacks, serum uric acid should be measured regularly during the course of treatment.
4. Interference with diagnosis: taking this product can increase the measured value of blood uric acid concentration.
5. If gastrointestinal irritation occurs, this product can be taken with food. Taking a daily dose in divided doses may not reach the effective blood concentration, so the daily dose of this product should be taken once.
[Medicine for pregnant and lactating women]
1. Since this product can pass through the placenta, the fetal blood concentration is about 30% of the maternal blood concentration. Animal experiments have shown that this product can cause malformations. Although no problems have been confirmed in humans, pregnant women should still ban this product. The pros and cons must be fully weighed when taking indications.
2. This product can be secreted into breast milk, and the concentration is similar to that of the mother's blood. Therefore, women who are breastfeeding should not use this product. If there is any indication for taking it, breastfeeding should be suspended.
【Children's Medication】
There is still a lack of clinical data for children under 13 years old. Because it is difficult to monitor changes in vision in children, this product is not suitable for children under 13 years old; the dosage for children over 13 years old is the same as that for adults.
【Medications for the Elderly】
Elderly patients should adjust the dosage according to renal function due to physiological decline of renal function.
【medicine interactions】
1. Aluminum salts, including DDI buffer can reduce the absorption of this product.
2. The combination of this product and verapamil can reduce the absorption of the latter.
3. Combined use with neurotoxic drugs can increase the neurotoxicity of this product, such as optic neuritis or peripheral neuritis.
4. Combined use with ethionamide can increase adverse reactions such as jaundice hepatitis and optic neuritis.
【Overdose】
1. Drug overdose is mainly manifested as the symptoms described in [Adverse Reactions]. In severe cases, permanent optic atrophy may occur.
2. Treatment of overdose
(1) Stop the drug.
(2) Symptomatic treatment: ① Vitamin B6, multivitamins and zinc-copper preparations can be used for patients with retrobulbar optic neuritis. ②To restore vision, you can choose dexamethasone 5mg, daily intravenous infusion or post-ball injection; torasulin 12.5mg, daily post-ball injection; hydrocortisone 200mg, daily intravenous injection. Prednisone 20mg can also be taken orally, 2 to 3 times a day. Give vitamins at the same time. During the recovery period, acupuncture treatment can be given, oral dibazole, niacin, etc., or placental tissue fluid intramuscular injection daily. ③ Perform hemodialysis and peritoneal dialysis when necessary.
【Pharmacology and Toxicology】
1. Pharmacology This product is a synthetic antibacterial anti-tuberculosis drug. It has strong activity against bacteria in the growth and reproduction phase, and has little effect on bacteria in the stationary phase. It has high antibacterial activity against various types of mycobacteria. There is no cross-resistance phenomenon between this product and other drugs by Mycobacterium tuberculosis.
The mechanism of action of this product has not been fully elucidated. It may be related to inhibiting the metabolism of sensitive bacteria, inhibiting the synthesis of RNA, and interfering with the protein metabolism of Mycobacterium tuberculosis, leading to the death of the bacteria.
2. Toxicology This product can cause cleft palate, brain exposure and spinal deformities in mouse experiments at high doses; it can cause mild cervical vertebra deformities in rat experiments; it can cause cyclops, short limbs and cleft palate in rabbit experiments Wait.
【Pharmacokinetics】
After oral administration, this product is absorbed by the gastrointestinal tract by 75% to 80%, and the peak time is 2 to 4 hours. It is widely distributed in various tissues in the body, and can be concentrated in red blood cells (the concentration in red blood cells can reach 2 to 3 times the blood concentration), kidney, lung, saliva and urine, and the concentration in pleural fluid and ascites is extremely low. The drug concentration in the cerebrospinal fluid is about 20% to 80% of the blood drug concentration, the apparent volume of distribution (Vd) is 1.6 to 3.9 L/kg, and the protein binding rate is about 10% to 30%. The blood elimination half-life (T1/2β) is 2.5 to 4 hours, and it can be extended to 7 to 15 hours for patients with impaired renal function, so the dosage should be adjusted. About 10% to 20% of this product is metabolized in the liver. This product is excreted through glomerular filtration and renal tubular secretion. After administration, about 50% to 90% of the drug is excreted in the kidney within 24 hours as a prototype, and about 15% is Inactive metabolites, renal clearance rate (ClR) is 5.93~8.45ml/min/kg. Approximately 20% are excreted in the feces as they are. The drug concentration in breast milk is approximately equivalent to the maternal blood drug concentration. Hemodialysis and peritoneal dialysis can remove this product.
【Storage】
Shade, seal, and store in a dry place.
【package】
Packed in plastic bottle, 100 capsules/bottle.
【Validity Period】
24 months
【Executive Standard】
"Chinese Pharmacopoeia" 2015 Edition Two
【Approval Number】
National Medicine Standard H21021909
【manufacturer】
Company Name: Shenyang Hongqi Pharmaceutical Co., Ltd.
Production address: No. 6, Xinluo Street, Hunnan New District, Shenyang City
Postal Code: 110179
Phone number: (024) 23786260 23786261
Fax number: (024) 23786263
Website: www.hongqipharma.com
Scan the QR code to read on your phone
Scan the QR code to read on your phone
Next
None
相关产品


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263