
"Anti-tuberculosis fixed-dose compound preparation" application pilot project summary meeting and promotion seminar in designated tuberculosis hospitals was successfully held
- Categories:Company news
- Author:
- Origin:
- Time of issue:2022-08-04
- Views:0
(Summary description)
"Anti-tuberculosis fixed-dose compound preparation" application pilot project summary meeting and promotion seminar in designated tuberculosis hospitals was successfully held
(Summary description)
- Categories:Company news
- Author:
- Origin:
- Time of issue:2022-08-04
- Views:0
In order to actively implement the "Technical Specifications for Tuberculosis Prevention and Control in China (2020 Edition)", first-line anti-tuberculosis drugs should be used for treatment of rifampicin-sensitive pulmonary tuberculosis patients without special circumstances, and anti-tuberculosis fixed-dose combination preparations (FDC) are recommended for treatment. requirements, improve the compliance of pulmonary tuberculosis patients, and reduce the incidence of drug-resistant tuberculosis. In response to the difficulty of using FDC in designated provincial and municipal tuberculosis hospitals, in April 2021, the China Antituberculosis Association launched the "Anti-tuberculosis Drug Fixed-dose Compound (FDC)" in five provinces including Jiangsu, Shandong, Jiangxi, Sichuan and Guizhou. Feasibility Pilot Project for Promotion and Use in Tuberculosis Specialized Hospitals”, to further understand the current status and feasibility of FDC use in provincial and municipal tuberculosis designated hospitals. In order to summarize the experience of the pilot project in a timely manner, introduce typical cases of the correct handling of the problems encountered in the clinical use of FDC, improve the understanding of the use of FDC, and accelerate the promotion and use of FDC in designated tuberculosis hospitals at all levels across the country, the China Antituberculosis Association in 2022. On July 28, a summary meeting and a seminar on promotion and application of FDC in designated TB hospitals were held offline and online. The meeting invited Zhao Yanlin, director of the Tuberculosis Prevention and Control Center of the Chinese Center for Disease Control and Prevention, deputy director Chen Mingting, Xu Caihong, director of the comprehensive business department, Zhou Lin, director of the patient care department, Liu Eryong, deputy director, Wang Ni, deputy director of the application technology department, and vice chairman of the China Antituberculosis Association. and Secretary-General Cheng Shiming, Deputy Secretary-General Fan Haiying, Ding Weimin, Director of the Endoscopy Center, Beijing Chest Hospital Affiliated to Capital Medical University, Lu Yu, Director of the Pharmacology Research Office, and Zhu Limei, Deputy Director of the Institute of Chronic Infectious Disease Control, Jiangsu Provincial Center for Disease Control and Prevention , Zeng Yi, Director of the Tuberculosis Department of Nanjing Second Hospital, Wu Guihui, Director of the Tuberculosis Department of Chengdu Public Health Clinical Medical Center, Ye Jiaqing, Tuberculosis Prevention and Control Institute of Jiangxi Provincial Center for Disease Control and Prevention, Geng Hong, Director of Prevention and Control Department of Shandong Provincial Public Health Clinical Center, Guizhou Guo Xiaohong, Vice President of the Third People's Hospital of Liupanshui City, Yang Rui, Chief Physician of the Tuberculosis Prevention and Control Institute of Yunnan Provincial Center for Disease Control and Prevention, and Shenyang Hongqi Pharmaceutical Co., Ltd. Representatives of the company and Zhejiang Su Ke'an Pharmaceutical Co., Ltd., a member of the enterprise director unit of the China Antituberculosis Association, attended the meeting. At the same time, 11,604 experts and scholars participated in the meeting online through the Internet platform of the Home of Science and Technology Workers.

Panorama of Beijing venue
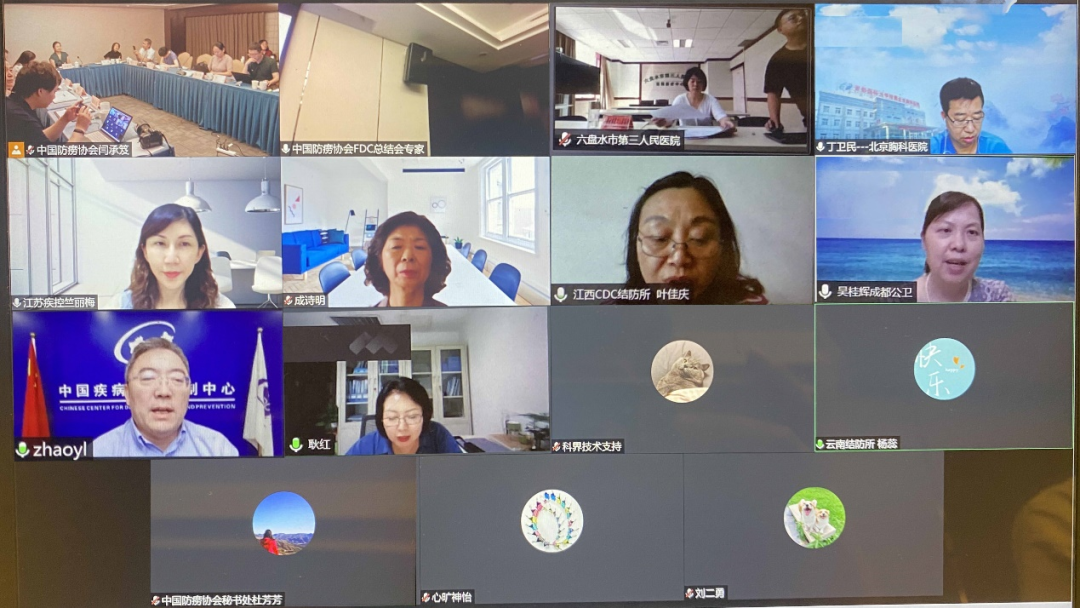
Panorama of online meeting
Director Zhao Yanlin pointed out in his speech that FDC has shown in a large number of applied studies that FDC can improve the compliance of patients with treatment. The simultaneous use of FDC can ensure the combination of drugs, thereby controlling the occurrence of drug-resistant tuberculosis.

Zhao Yanlin, Director of the Tuberculosis Prevention and Control Center of the Chinese Center for Disease Control and Prevention, delivered a speech
"Report on the Feasibility Pilot Project of the Promotion and Use of Fixed-dose Compounds of Anti-tuberculosis Drugs in Designated Tuberculosis Hospitals"
The keynote report on the pilot study of the conference was chaired by Chen Mingting, Deputy Director of the Nuclear Disease Prevention and Control Center of the Chinese Center for Disease Control and Prevention, and Wang Nizuo, Deputy Director of the Applied Technology Department of the Center for Disease Control and Prevention of the Chinese Center for Disease Control and Prevention Feasibility Pilot Project Report for Use. The report pointed out that it is necessary to further promote the use of anti-tuberculosis FDC in the country and maintain a high utilization rate, improve the drug supply management system of medical and preventive cooperation, and recommend increasing publicity to improve patients' awareness of standardized treatment, and gradually promote the use of video supervision, New tools such as electronic pill box supervision assist patients with medication management and improve patient acceptance and treatment compliance.

Tuberculosis Prevention and Control Center, Chinese Center for Disease Control and Prevention
Deputy Director Chen Mingting presided over the keynote report on the pilot study

Department of Applied Technology, TB Control Center, Chinese Center for Disease Control and Prevention
Deputy Director Wang Ni
"Expert Consensus Interpretation and Use Case Analysis of Clinical Use of Anti-TB FDC"
Zhou Lin, Director of the Patient Care Department of the Tuberculosis Prevention and Control Center of the Chinese Center for Disease Control and Prevention, made a report on "Expert Consensus Interpretation and Use Case Analysis for Clinical Use of Anti-TB FDC". The introduction of fixed-dose compound preparations, the significance of using FDC, the application of FDC in my country, the possible drugs and treatment of FDC adverse reactions, and clinical case analysis were introduced, and corresponding treatment opinions and suggestions were put forward in turn.

Tuberculosis Prevention and Control Center, Chinese Center for Disease Control and Prevention
Zhou Lin, Director of Patient Care Department
"Re-understanding FDC: FDC's Evidence-Based and Clinical Drug Experience Sharing"
Ding Weimin, director of the Endoscopy Center of Beijing Chest Hospital Affiliated to Capital Medical University, and Zhu Limei, deputy director of the Institute of Chronic Infectious Disease Control, Jiangsu Provincial Center for Disease Control and Prevention, co-chaired the pilot research experience sharing. Experts from FDC pilot units in 5 different provinces The pilot research experience was shared in turn, and questions and discussions were conducted with online experts and scholars.
 |
 |
|
Ding Weimin, director of the Endoscopy Center, Beijing Chest Hospital Affiliated to Capital Medical University, and Zhu Limei, deputy director of the Institute of Chronic Infectious Disease Control, Jiangsu Center for Disease Control and Prevention, co-chaired the meeting. |
|

Zeng Yi, Director of the Tuberculosis Department of Nanjing Second Hospital
"Chengdu Public Health FDC Clinical Application Experience Sharing"
Wu Guihui, Director of the Tuberculosis Department of Chengdu Public Health Clinical Medical Center

"Summary of Jiangxi Province FDC Promotion Pilot Project"
Ye Jiaqing, Institute of Tuberculosis Prevention and Control, Jiangxi Provincial Center for Disease Control and Prevention
"Experience sharing of key measures to increase the proportion of individual use of FDC in Shandong Province"
Geng Hong, Director of Prevention and Control Division of Shandong Provincial Public Health Clinical Center
"Pharmaceutical research on fixed-dose compound preparations of anti-tuberculosis drugs"
Liu Eryong, Deputy Director of the Patient Care Department of the Tuberculosis Prevention and Control Center of the Chinese Center for Disease Control and Prevention, and Fan Haiying, Deputy Secretary-General of the China Tuberculosis Prevention Association, presided over the FDC Pharmacy Theory, Application Quality Control and Policy Interpretation Forum, from the Pharmacy Laboratory of Beijing Chest Hospital Affiliated to Capital Medical University Director Lu Yu made reports from the rationality, pharmacodynamics and bioequivalence studies of FDC specifications. Director Lu said that the bioequivalence of FDC is qualified and the use of FDC with reasonable specifications can significantly reduce the risk of monotherapy and effectively prevent the emergence of drug-resistant tuberculosis; at the same time, FDC can reduce the number of pills taken by patients and greatly increase the number of pills taken by patients. Compliance, to ensure clinical efficacy.

Tuberculosis Prevention and Control Center, Chinese Center for Disease Control and Prevention
Liu Eryong, Deputy Director of the Patient Care Department, presided over the meeting

Beijing Chest Hospital Affiliated to Capital Medical University
Lu Yu, Director of the Pharmacy Research Office
"Comprehensive Quality Control of Tuberculosis Prevention and Control"
Xu Caihong, director of the comprehensive business department of the Chinese Center for Disease Control and Prevention, made a report on the comprehensive quality control of tuberculosis prevention and control. From the perspective of comprehensive quality control, the 3 parts used by FDC are explained.

Chinese Center for Disease Control and Prevention
Xu Caihong, Director of Integrated Business Department
"Interpretation and Application Practice of FDC Use Policy in Yunnan Province"
Yang Rui, the chief physician of the Tuberculosis Prevention and Control Institute of Yunnan Provincial Center for Disease Control and Prevention, gave a report on the interpretation and application of FDC use policy in Yunnan Province. The report analyzed the investigation and research on anti-tuberculosis drug prescriptions in Yunnan Province, and introduced measures to improve the use rate of FCD. , including incorporating FDC into provincial prevention and control planning, provincial overall planning, ensuring uninterrupted supply of drugs, inclusion in key work task indicators, special training and regular supervision, etc.

Yang Rui, Physician in Charge of Tuberculosis Prevention and Control Institute of Yunnan Provincial Center for Disease Control and Prevention

Shenyang Hongqi Pharmaceutical Co., Ltd., a member of Fosun Pharma
Lv Wenhua, General Manager of PHS Division, delivering a speech
Shenyang Hongqi Pharmaceutical Co., Ltd. was established in 1964, and has been focusing on the research and development, production, sales and service of anti-tuberculosis drugs since the 1980s. In 2010, Hongqi Pharmaceutical became a member of Shanghai Fosun Pharmaceutical Co., Ltd. For a long time, with the mission of "letting human beings no longer be troubled by tuberculosis" and the vision of "creating a world-class anti-tuberculosis drug pharmaceutical company", Hongqi Pharmaceutical has continued to deepen its efforts in scientific and technological innovation and pharmaceutical research and development. Prevention and control business. The continuous and uninterrupted supply and management of high-quality anti-TB medicines is an important part of patient-centred treatment management and care in the WHO End-TB Strategy (End-TB). Hongqi Pharmaceutical is currently one of the largest anti-tuberculosis drug research and development and production bases in China. At the same time, as the China Antituberculosis Association’s anti-tuberculosis drug supply coordination reserve, in order to ensure the continuous supply of high-quality anti-tuberculosis drugs, Hongqi Pharmaceutical will pass the national level of the Ministry of Industry and Information Technology in 2021. The green factory audit has become a benchmark for green manufacturing; the company's existing first-line anti-tuberculosis drugs have passed the national generic drug consistency evaluation; in order to bring accessible and most advanced treatment drugs to drug-resistant tuberculosis patients, Hongqi Pharmaceutical actively promotes second-line anti-tuberculosis drugs Drug localization innovation, carry out the real-world research project of NIX program for multidrug-resistant patients, and accelerate the domestic launch of PA-824; in terms of raw materials, strategic cooperation with domestic advanced enterprises such as Pro Pharmaceuticals has been reached on the research and development of raw materials and intermediates, and the whole chain Guarantee the supply of high-quality medicines. Lv Wenhua, general manager of the business department, introduced the "Double Thousand Actions" project carried out by the China Antituberculosis Public Welfare Fund project in recent years. As a responsible and responsible pharmaceutical manufacturer, we actively fulfill our social responsibilities. In 2016, Hongqi Pharma, together with China Antituberculosis Association and Fosun Foundation, jointly launched a relief project for poor tuberculosis patients - "Double Thousand Actions". Up to now, the project has been in operation for five years, with a total investment of more than 5 million yuan, helping more than 5,000 poor tuberculosis patients. In 2021, the "End Tuberculosis Health Cultivation" project will be launched with the China Tuberculosis Prevention Association, aiming to strengthen the standardized diagnosis and treatment of tuberculosis and the training of talents in the field of anti-tuberculosis. Fosun Pharma--Shenyang Hongqi Pharmaceutical Co., Ltd. will actively cooperate with the China Antituberculosis Association to carry out various tasks, coordinate and improve the national tuberculosis prevention and control service network and professional teams, effectively assist in the implementation of China's tuberculosis control strategy, increase the proportion of standard tuberculosis diagnosis and treatment, and effectively reduce tuberculosis. The economic burden of patients, improve the health level of the people, and promote the comprehensive and coordinated development of the economy and society and the realization of the Healthy China strategy. Contribute to the realization of the goal of ending the TB epidemic.

Cheng Shiming, vice chairman and secretary general of China Tuberculosis Prevention Association, made a summary of the meeting
Cheng Shiming, vice chairman and secretary general of the China Antituberculosis Association, made a summary of the meeting. She said that the online and offline experts of this meeting discussed the practical application of FDC through various forms such as theoretical teaching, experience sharing and case discussion. And put forward very good suggestions. I hope to investigate the current situation and existing problems of FDC use in the future, strengthen the training of medical staff, expand the popularization and education of patients, put funds in place, strengthen policy development and formulation, and do a good job in FDC. Use evaluation and promotion. Experts inspire each other and work together, and the association will work with scientists and anti-tuberculosis companies to achieve the goal of ending tuberculosis as soon as possible.

Scan the QR code to read on your phone


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263


