Number of views:
1000
Praziquantel Tablets
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Quantity
-
+
Stock:
Category
1
Product description
Parameters
 Praziquantel Tablets Instructions
Praziquantel Tablets Instructions
Please read the instructions carefully and use under the guidance of a doctor
Patients with ocular cysticercosis should not use this product
【Drug Name】
Generic name: Praziquantel tablets
English name: Praziquantel Tablets
Chinese Pinyin: Bikuitong Piɑn
【Ingredients】
The main ingredient of this product is praziquantel.
The chemical name is: 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-α]isoquinolin-4-one
Chemical Structure:
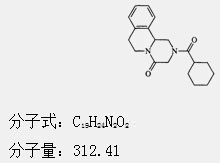
【Properties】
This product is a white piece.
【Indications】
It is a broad-spectrum anti-fluke and tapeworm drug. It is suitable for all kinds of schistosomiasis, clonorchiasis, paragonimiasis, ginger worm disease, tapeworm disease and cysticercosis.
【specification】
0.2g
【Dosage】
1. Treatment of trematodiasis
① Schistosomiasis: All kinds of chronic schistosomiasis are treated with a total dose of 60mg/kg for 1 to 2 days, and the daily dose is divided into 2 to 3 meals between meals. The total dose of acute schistosomiasis is 120mg/kg, divided into 2 to 3 times a day for 4 days. Those who weigh more than 60kg will be calculated as 60kg.
② Clonorchis sinensis: The total dose is 210mg/kg, 3 times a day for 3 days.
③ Paragonimiasis: 25mg/kg, 3 times a day for 3 days.
④ Ginger worm disease: 15mg/kg, take a meal.
2. Treatment of tapeworm disease
①Taeniasis of beef and pork: 10mg/kg, take it in the morning, and take magnesium sulfate 1 hour later.
② Hymenoderma brevisiae and Schizocephalys broad-segmented tapeworm disease: 25mg/kg, take a meal.
3. Treatment of cysticercosis The total dose is 120-180mg/kg, divided into 3 to 5 days, and the daily amount is divided into 2 to 3 times.
【Adverse reactions】
1. Systemic damage: fatigue, sore limbs, eosinophilia, in addition, there are reports in the literature that this product causes anaphylactic shock.
2. Cardiovascular system: palpitations, chest tightness, ECG showing T wave changes and extrasystole, supraventricular tachycardia, atrial fibrillation.
3. Digestive system: nausea, abdominal pain, diarrhea, elevated transaminase, gastrointestinal bleeding.
4. Skin and its accessories: rash, itching, allergic purpura.
5. Nervous system: dizziness, headache.
6. Other: induce mental disorders.
【Taboo】
1. Disabled for patients with ocular cysticercosis.
2. It is contraindicated in patients who are allergic to this drug or pharmaceutical excipients.
3. It is forbidden to simultaneously use strong cytochrome P450 inducers, such as rifampicin.
【Precautions】
1. Treatment of parasites in the tissues such as schistosomiasis, lung fluke, cysticercosis, etc. Because the parasites are killed and release a large amount of antigenic substances, they can cause fever, eosinophilia, skin rashes, etc., and occasionally can cause Anaphylactic shock must be observed.
2. Patients with cerebral cysticercosis need to be hospitalized, and supplemented with treatment measures to prevent and treat brain edema and reduce high intracranial pressure (using dexamethasone and dehydrating agents) or prevent and treat status epilepticus to prevent accidents.
3. When combined with ocular cysticercosis, the worms must be surgically removed and then treated with medication.
4. Use with caution in patients with severe heart, liver and kidney and those with a history of mental illness.
5. Those who have obvious dizziness, drowsiness and other neurological reactions, do not drive or operate machinery during treatment and within 24 hours after stopping the drug.
6. When cysticercosis is used to expel tapeworms, recessive cerebral cysticercosis should be excluded to avoid accidents.
7. This product will aggravate the central nervous system disease caused by schistosomiasis, so this drug should not be given to patients with a history of epilepsy and/or patients with other symptoms of the underlying central nervous system, such as cysticercosis subcutaneous nodules.
8. Patients receiving rifampicin treatment but urgently in need of parasitic drug treatment should consider using other preparations for treatment. However, if treatment with praziquantel is necessary, rifampin should be discontinued for 4 weeks before administration. One day after the completion of praziquantel treatment, the treatment of rifampicin can be resumed.
[Medicine for pregnant and lactating women]
During the period of taking the drug, breastfeeding women should not breastfeed until 72 hours after the drug is stopped.
【Children's Medication】
The experiment has not been carried out and there is no reliable reference.
【Medications for the Elderly】
The experiment has not been carried out and there is no reliable reference.
【medicine interactions】
The experiment has not been carried out and there is no reliable reference.
【Overdose】
The experiment has not been carried out and there is no reliable reference.
【Pharmacology and Toxicology】
This product is effective against schistosomiasis, tapeworm, cysticercosis, clonorchis sinensis, lung fluke and ginger fluke. There are two main pharmacological effects on the parasite:
1. The worm body muscles undergo tonic contraction and produce spastic paralysis. The body tension of schistosomes increased only 20 seconds after exposure to low concentrations of praziquantel. When the drug concentration reached 1 mg/L or more, the worms contracted immediately. The muscle contraction of the worm body may be related to the increase of the permeability of the worm body cell membrane by praziquantel and the loss of intracellular calcium ions.
2. Cortex damage and host immune function participation: Praziquantel has a rapid and obvious damage effect on the cortex of the worm body, causing the syncytial skin to swell, appearing vacuoles, forming bullae, protruding the body surface, and eventually epidermal erosion and ulcer , Almost all the secretions disappeared, and the circular muscles and longitudinal muscles also quickly dissolved. In the host, vacuolar degeneration of the outer skin of the worm can be seen 15 minutes after taking the medicine. After the cortex is damaged, it affects the body's absorption and excretion function, and more importantly, its body surface antigen is exposed, which makes it vulnerable to host immune attack. A large number of eosinophils attach to the skin lesions and invade, which promotes the death of the body. In addition, praziquantel can also cause secondary changes, depolarize the surface membrane of the worm, and significantly reduce the activity of cortical alkaline phosphatase, resulting in inhibition of glucose uptake and depletion of endogenous glycogen. Praziquantel can also inhibit the synthesis of nucleic acid and protein.
【Pharmacokinetics】
The absorption is rapid after oral administration, and more than 80% of the drug can be absorbed from the intestine. The peak blood drug reaches about 1 hour, and the drug is metabolized quickly after entering the liver, mainly forming hydroxyl metabolites, and only a very small amount of unmetabolized original drug enters the systemic circulation. The concentration in portal vein blood can be more than 10 times higher than that in peripheral vein blood. The concentration of cerebrospinal fluid is about 15% to 20% of the blood drug concentration. After taking the drug, the drug concentration in the breast milk is equivalent to 25% of the serum. The peak blood drug after oral administration of 10-15 mg/kg is about 1 mg/L. The drug is mainly distributed in the liver, followed by the kidneys, lungs, pancreas, adrenal glands, pituitary gland, salivary glands, etc. It rarely passes through the placenta and has no organ-specific accumulation. The half-life (t1/2) is 0.8 to 1.5 hours, and the half-life (t1/2) of its metabolites is 4 to 5 hours. It is mainly excreted in the form of metabolites by the kidneys, 72% is excreted within 24 hours, and 80% is excreted within 4 days.
【Storage】
Shading, sealed and preserved.
【Packing】
Aluminum-plastic blister packaging, 18 pieces/plate, carton packaging, 2 plates/box;
Aluminum-plastic blister packaging, 36 pieces/plate, carton packaging, 10 plates/box;
Plastic bottle packaging, 100 tablets/bottle, carton packaging, 1 bottle/box;
Packed in plastic bottle, 1000 tablets/bottle.
【Validity Period】
24 months
【Executive Standard】
"Chinese Pharmacopoeia" 2015 Edition Two
【Approval number】
National Medicine Standard H20093670
【manufacturer】
Company Name: Shenyang Hongqi Pharmaceutical Co., Ltd.
Production address: No. 6, Xinluo Street, Hunnan New District, Shenyang City
Postal Code: 110179
Phone number: (024) 23786260 23786261
Fax number: (024) 23786263
Website: www.hongqipharma.com
Scan the QR code to read on your phone
Scan the QR code to read on your phone
Previous
Inosine Tablets
Next
None
相关产品


Copyright hongqipharma.com All rights reserved Powered by:300.cn Shenyang 辽icp备12005917号-1 (辽)-非经营性-2018-0012
Add:Shenyang Hunnan New District envelope 6th Street Tel:024-23786268-8012 Fax:024-23786263